Chapter 7: THE CARDIOVASCULAR SYSTEM: BLOOD VESSELS AND CIRCULATION
Introduction
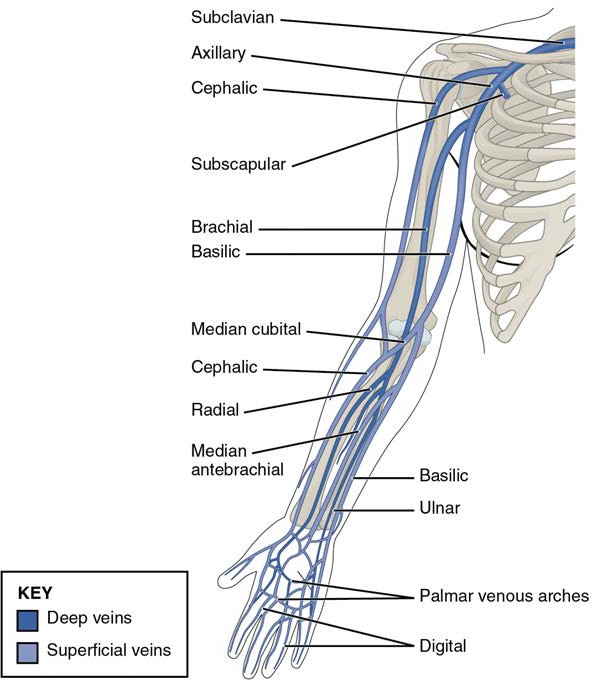
Figure 20.1 Blood Vessels While most blood vessels are located deep from the surface and are not visible, the superficial veins of the upper limb provide an indication of the extent, prominence, and importance of these structures to the body. (credit: Colin Davis)
Chapter Objectives
Type your learning objectives here.
- Compare and contrast the anatomical structure of arteries, arterioles, capillaries, venules, and veins
- Accurately describe the forces that account for capillary exchange
- List the major factors affecting blood flow, blood pressure, and resistance
- Describe how blood flow, blood pressure, and resistance interrelate
- Discuss how the neural and endocrine mechanisms maintain homeostasis within the blood vessels
- Describe the interaction of the cardiovascular system with other body systems
- Label the major blood vessels of the pulmonary and systemic circulations
- Identify and describe the hepatic portal system
In this chapter, you will learn about the vascular part of the cardiovascular system, that is, the vessels that transport blood throughout the body and provide the physical site where gases, nutrients, and other substances are exchanged with body cells. When vessel functioning is reduced, blood-borne substances do not circulate effectively throughout the body. As a result, tissue injury occurs, metabolism is impaired, and the functions of every bodily system are threatened.
[20.1] Structure and Function of Blood Vessels
Learning Objectives
By the end of this section, you will be able to:
- Compare and contrast the three tunics that make up the walls of most blood vessels
- Distinguish between elastic arteries, muscular arteries, and arterioles on the basis of structure, location, and function
- Describe the basic structure of a capillary bed, from the supplying metarteriole to the venule into which it drains
- Explain the structure and function of venous valves in the large veins of the extremities
Blood is carried through the body via blood vessels. An artery is a blood vessel that carries blood away from the heart, where it branches into ever-smaller vessels. Eventually, the smallest arteries, vessels called arterioles, further branch into tiny capillaries, where nutrients and wastes are exchanged, and then combine with other vessels that exit capillaries to form venules, small blood vessels that carry blood to a vein, a larger blood vessel that returns blood to the heart.
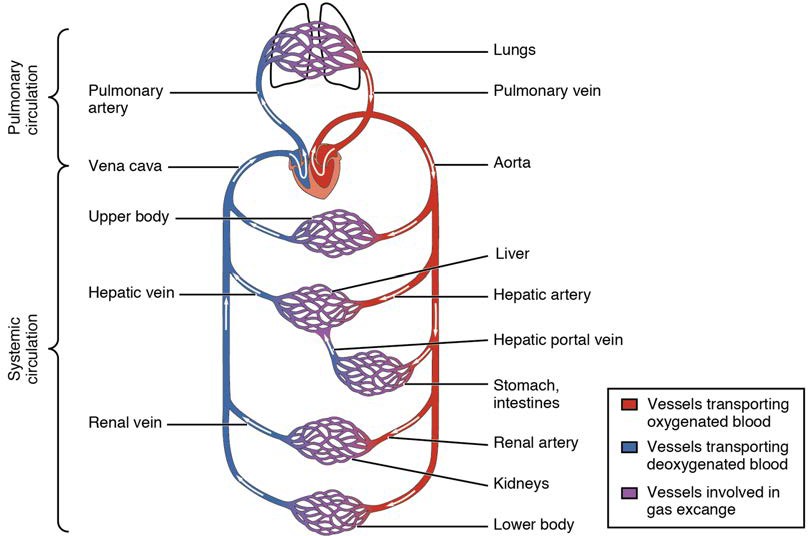
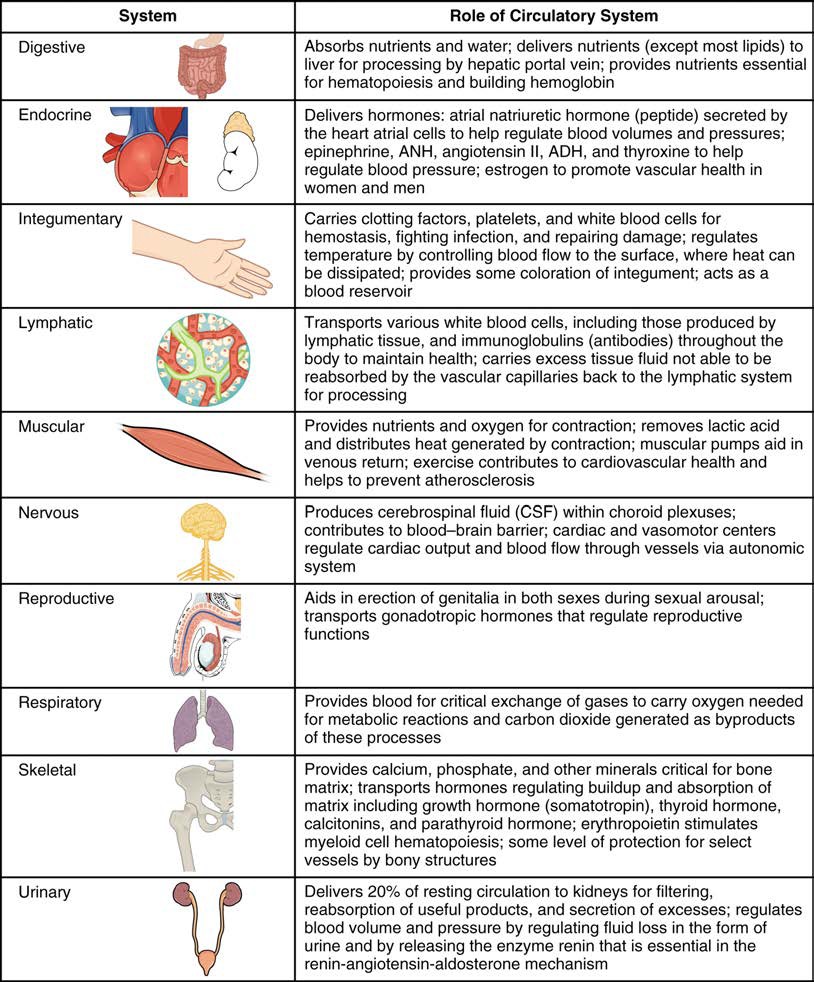
Arteries and veins transport blood in two distinct circuits: the systemic circuit and the pulmonary circuit (Figure 20.2). Systemic arteries provide blood rich in oxygen to the body’s tissues. The blood returned to the heart through systemic veins has less oxygen, since much of the oxygen carried by the arteries has been delivered to the cells. In contrast, in the pulmonary circuit, arteries carry blood low in oxygen exclusively to the lungs for gas exchange. Pulmonary veins then return freshly oxygenated blood from the lungs to the heart to be pumped back out into systemic circulation. Although arteries and veins differ structurally and functionally, they share certain features.
Figure 20.2 Cardiovascular Circulation The pulmonary circuit moves blood from the right side of the heart to the lungs and back to the heart. The systemic circuit moves blood from the left side of the heart to the head and body and returns it to the right side of the heart to repeat the cycle. The arrows indicate the direction of blood flow, and the colors show the relative levels of oxygen concentration.
[20.1.1] Shared Structures
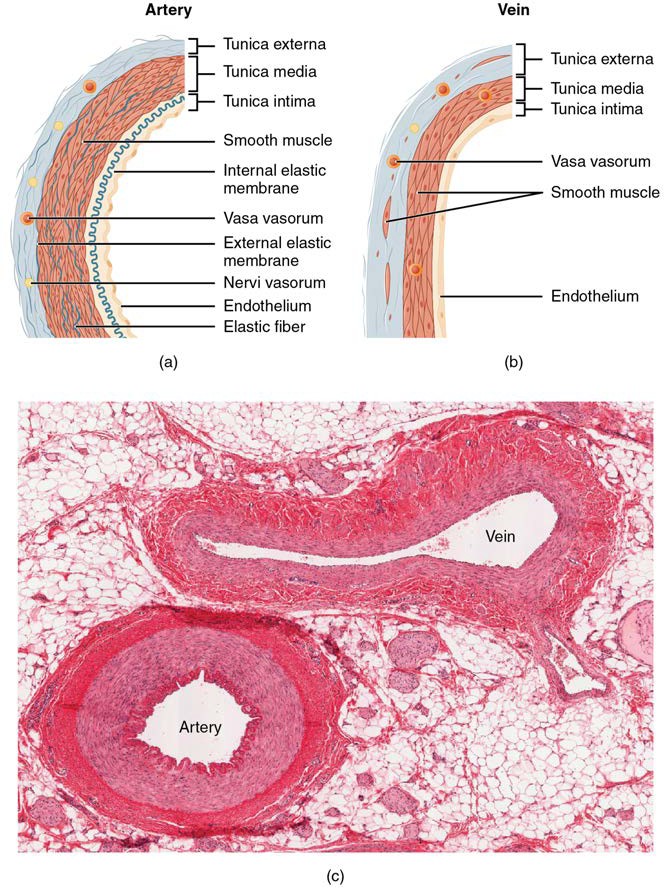
Different types of blood vessels vary slightly in their structures, but they share the same general features. Arteries and arterioles have thicker walls than veins and venules because they are closer to the heart and receive blood that is surging at a far greater pressure (Figure 20.3). Each type of vessel has a lumen—a hollow passageway through which blood flows. Arteries have smaller lumens than veins, a characteristic that helps to maintain the pressure of blood moving through the system. Together, their thicker walls and smaller diameters give arterial lumens a more rounded appearance in cross section than the lumens of veins.
Figure 20.3 Structure of Blood Vessels (a) Arteries and (b) Veins share the same general features, but the walls of arteries are much thicker because of the higher pressure of the blood that flows through them. (c) Muscular or medium sized artery and companion vein. (Michigan Histology and Virtual Miscroscopy Learning Resources)
By the time blood has passed through capillaries and entered venules, the pressure initially exerted upon it by heart contractions has diminished. In other words, in comparison to arteries, venules and veins withstand a much lower pressure from the blood that flows through them. Their walls are considerably thinner and their lumens are correspondingly larger in diameter, allowing more blood to flow with less vessel resistance. In addition, many veins of the body, particularly those of the limbs, contain valves that assist the unidirectional flow of blood toward the heart. This is critical because blood flow becomes sluggish in the extremities, as a result of the lower pressure and the effects of gravity.
The walls of arteries and veins are largely composed of living cells and their products (including collagenous and elastic fibers); the cells require nourishment and produce waste. Since blood passes through the larger vessels relatively quickly, there is limited opportunity for blood in the lumen of the vessel to provide nourishment to or remove waste from the vessel’s cells. Further, the walls of the larger vessels are too thick for nutrients to diffuse through to all of the cells. Larger arteries and veins contain small blood vessels within their walls known as the vasa vasorum—literally “vessels of the vessel”—to provide them with this critical exchange. Since the pressure within arteries is relatively high, the vasa vasorum must function in the outer layers of the vessel (see Figure 20.3) or the pressure exerted by the blood passing through the vessel would collapse it, preventing any exchange from occurring. The lower pressure within veins allows the vasa vasorum to be located closer to the lumen. The restriction of the vasa vasorum to the outer layers of arteries is thought to be one reason that arterial diseases are more common than venous diseases, since its location makes it more difficult to nourish the cells of the arteries and remove waste products. There are also minute nerves within the walls of both types of vessels that control the contraction and dilation of smooth muscle. These minute nerves are known as the nervi vasorum.
Both arteries and veins have the same three distinct tissue layers, called tunics (from the Latin term tunica), for the garments first worn by ancient Romans; the term tunic is also used for some modern garments. From the most interior layer to the outer, these tunics are the tunica intima, the tunica media, and the tunica externa (see Figure 20.3). Table 20.1 compares and contrasts the tunics of the arteries and veins.
Comparison of Tunics in Arteries and Veins
| Arteries | Veins | |
| General appearance | Thick walls with small lumens Generally appear rounded |
Thin walls with large lumens Generally appear flattened |
| Tunica intima | Endothelium usually appears wavy due to constriction of smooth muscle Internal elastic membrane present in larger vessels |
Endothelium appears smooth Internal elastic membrane absent |
| Tunica media | Normally the thickest layer in arteries Smooth muscle cells and elastic fibers predominate (the proportions of these vary with distance from the heart) External elastic membrane present in larger vessels |
Normally thinner than the tunica externa Smooth muscle cells and collagenous fibers predominate Nervi vasorum and vasa vasorum present External elastic membrane absent |
| Tunica externa | Normally thinner than the tunica media in all but the largest arteries Collagenous and elastic fibers Nervi vasorum and vasa vasorum present |
Normally the thickest layer in veins Collagenous and smooth fibers predominate Some smooth muscle fibers Nervi vasorum and vasa vasorum present |
Table 20.1
Tunica Intima
The tunica intima (also called the tunica interna) is composed of epithelial and connective tissue layers. Lining the tunica intima is the specialized simple squamous epithelium called the endothelium, which is continuous throughout the entire vascular system, including the lining of the chambers of the heart. Damage to this endothelial lining and exposure of blood to the collagenous fibers beneath is one of the primary causes of clot formation. Until recently, the endothelium was viewed simply as the boundary between the blood in the lumen and the walls of the vessels. Recent studies, however, have shown that it is physiologically critical to such activities as helping to regulate capillary exchange and altering blood flow. The endothelium releases local chemicals called endothelins that can constrict the smooth muscle within the walls of the vessel to increase blood pressure. Uncompensated overproduction of endothelins may contribute to hypertension (high blood pressure) and cardiovascular disease.
Next to the endothelium is the basement membrane, or basal lamina, that effectively binds the endothelium to the connective tissue. The basement membrane provides strength while maintaining flexibility, and it is permeable, allowing materials to pass through it. The thin outer layer of the tunica intima contains a small amount of areolar connective tissue that consists primarily of elastic fibers to provide the vessel with additional flexibility; it also contains some collagenous fibers to provide additional strength.
In larger arteries, there is also a thick, distinct layer of elastic fibers known as the internal elastic membrane (also called the internal elastic lamina) at the boundary with the tunica media. Like the other components of the tunica intima, the internal elastic membrane provides structure while allowing the vessel to stretch. It is permeated with small openings that allow exchange of materials between the tunics. The internal elastic membrane is not apparent in veins. In addition, many veins, particularly in the lower limbs, contain valves formed by sections of thickened endothelium that are reinforced with connective tissue, extending into the lumen.
Under the microscope, the lumen and the entire tunica intima of a vein will appear smooth, whereas those of an artery will normally appear wavy because of the partial constriction of the smooth muscle in the tunica media, the next layer of blood vessel walls.
Tunica Media
The tunica media is the substantial middle layer of the vessel wall (see Figure 20.3). It is generally the thickest layer in arteries, and it is much thicker in arteries than it is in veins. The tunica media consists of layers of smooth muscle supported by connective tissue that is primarily made up of elastic fibers, most of which are arranged in circular sheets. Toward the outer portion of the tunic, there are also layers of longitudinal muscle. Contraction and relaxation of the circular muscles decrease and increase the diameter of the vessel lumen, respectively. Specifically in arteries, vasoconstriction decreases blood flow as the smooth muscle in the walls of the tunica media contracts, making the lumen narrower and increasing blood pressure. Similarly, vasodilation increases blood flow as the smooth muscle relaxes, allowing the lumen to widen and blood pressure to drop. Both vasoconstriction and vasodilation are regulated in part by small vascular nerves, known as nervi vasorum, or “nerves of the vessel,” that run within the walls of blood vessels. These are generally all sympathetic fibers, although some trigger vasodilation and others induce vasoconstriction, depending upon the nature of the neurotransmitter and receptors located on the target cell. Parasympathetic stimulation does trigger vasodilation as well as erection during sexual arousal in the external genitalia of both sexes. Nervous control over vessels tends to be more generalized than the specific targeting of individual blood vessels. Local controls, discussed later, account for this phenomenon. Hormones including catecholamines and antidiuretic hormone in combination with local chemicals such as neurotransmitters control blood vessel diameter in response to blood pressure. Release of these hormones is regulated by the Autonomic Nervous System (ANS), in addition to local chemical release to control overall blood vessel diameter, cardiac output and blood composition. Together, these neural and chemical mechanisms reduce or increase blood flow in response to changing body conditions, from exercise to hydration. Regulation of both blood flow and blood pressure is discussed in detail later in this chapter.
The smooth muscle layers of the tunica media are supported by a framework of collagenous fibers that also binds the tunica media to the inner and outer tunics. Along with the collagenous fibers are large numbers of elastic fibers that appear as wavy lines in prepared slides. Separating the tunica media from the outer tunica externa in larger arteries is the external elastic membrane (also called the external elastic lamina), which also appears wavy in slides. This structure is not usually seen in smaller arteries, nor is it seen in veins.
Tunica Externa
The outer tunic, the tunica externa (also called the tunica adventitia), is a substantial sheath of connective tissue composed primarily of collagenous fibers. Some bands of elastic fibers are found here as well. The tunica externa in veins also contains groups of smooth muscle fibers. This is normally the thickest tunic in veins and may be thicker than the tunica media in some larger arteries. The outer layers of the tunica externa are not distinct but rather blend with the surrounding connective tissue outside the vessel, helping to hold the vessel in relative position. If you are able to palpate some of the superficial veins on your upper limbs and try to move them, you will find that the tunica externa prevents this. If the tunica externa did not hold the vessel in place, any movement would likely result in disruption of blood flow.
[20.1.2] Arteries
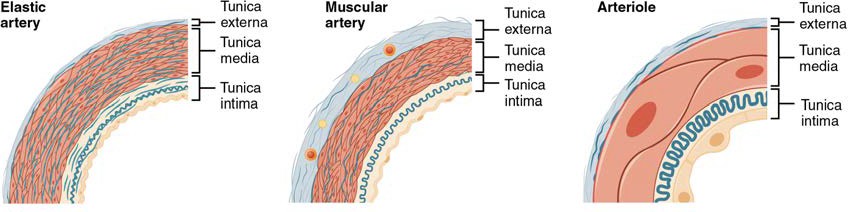
An artery is a blood vessel that conducts blood away from the heart. All arteries have relatively thick walls that can withstand the high pressure of blood ejected from the heart. However, those close to the heart have the thickest walls, containing a high percentage of elastic fibers in all three of their tunics. This type of artery is known as an elastic artery (Figure 20.4). Vessels larger than 10 mm in diameter are typically elastic. Their abundant elastic fibers allow them to expand, as blood pumped from the ventricles passes through them, and then to recoil after the surge has passed. If artery walls were rigid and unable to expand and recoil, their resistance to blood flow would greatly increase and blood pressure would rise to even higher levels, which would in turn require the heart to pump harder to increase the volume of blood expelled by each pump (the stroke volume) and maintain adequate pressure and flow. Artery walls would have to become even thicker in response to this increased pressure. The elastic recoil of the vascular wall helps to maintain the pressure gradient that drives the blood through the arterial system. An elastic artery is also known as a conducting artery, because the large diameter of the lumen enables it to accept a large volume of blood from the heart and conduct it to smaller branches.
Figure 20.4 Types of Arteries and Arterioles Comparison of the walls of an elastic artery, a muscular artery, and an arteriole is shown. In terms of scale, the diameter of an arteriole is measured in micrometers compared to millimeters for elastic and muscular arteries.
Farther from the heart, where the surge of blood has dampened, the percentage of elastic fibers in an artery’s tunica intima decreases and the amount of smooth muscle in its tunica media increases. The artery at this point is described as a muscular artery. The diameter of muscular arteries typically ranges from 0.1 mm to 10 mm. Their thick tunica media allows muscular arteries to play a leading role in vasoconstriction. In contrast, their decreased quantity of elastic fibers limits their ability to expand. Fortunately, because the blood pressure has eased by the time it reaches these more distant vessels, elasticity has become less important.
Notice that although the distinctions between elastic and muscular arteries are important, there is no “line of demarcation” where an elastic artery suddenly becomes muscular. Rather, there is a gradual transition as the vascular tree repeatedly branches. In turn, muscular arteries branch to distribute blood to the vast network of arterioles. For this reason, a muscular artery is also known as a distributing artery.
[20.1.3] Arterioles
An arteriole is a very small artery that leads to a capillary. Arterioles have the same three tunics as the larger vessels, but the thickness of each is greatly diminished. The critical endothelial lining of the tunica intima is intact. The tunica media is restricted to one or two smooth muscle cell layers in thickness. The tunica externa remains but is very thin (see Figure 20.4).
With a lumen averaging 30 micrometers or less in diameter, arterioles are critical in slowing down—or resisting—blood flow and, thus, causing a substantial drop in blood pressure. Because of this, you may see them referred to as resistance vessels. The muscle fibers in arterioles are normally slightly contracted, causing arterioles to maintain a consistent muscle tone—in this case referred to as vascular tone—in a similar manner to the muscular tone of skeletal muscle. In reality, all blood vessels exhibit vascular tone due to the partial contraction of smooth muscle. The importance of the arterioles is that they will be the primary site of both resistance and regulation of blood pressure. The precise diameter of the lumen of an arteriole at any given moment is determined by neural and chemical controls, and vasoconstriction and vasodilation in the arterioles are the primary mechanisms for distribution of blood flow.
[20.1.4] Capillaries
A capillary is a microscopic channel that supplies blood to the tissues themselves, a process called perfusion. Exchange of gases and other substances occurs in the capillaries between the blood and the surrounding cells and their tissue fluid (interstitial fluid). The diameter of a capillary lumen ranges from 5–10 micrometers; the smallest are just barely wide enough for an erythrocyte to squeeze through. Flow through capillaries is often described as microcirculation.
The wall of a capillary consists of the endothelial layer surrounded by a basement membrane with occasional smooth muscle fibers. There is some variation in wall structure: In a large capillary, several endothelial cells bordering each other may line the lumen; in a small capillary, there may be only a single cell layer that wraps around to contact itself.
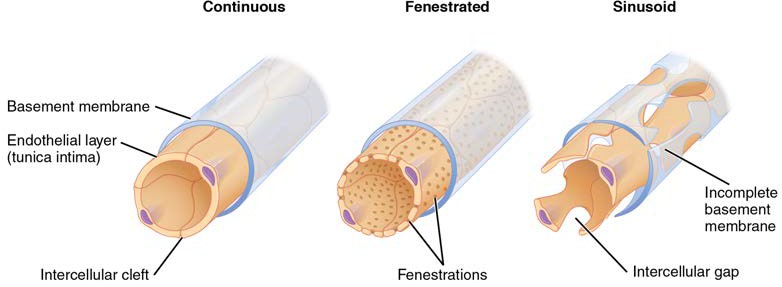
For capillaries to function, their walls must be leaky, allowing substances to pass through. There are three major types of capillaries, which differ according to their degree of “leakiness:” continuous, fenestrated, and sinusoid capillaries (Figure 20.5).
Continuous Capillaries
The most common type of capillary, the continuous capillary, is found in almost all vascularized tissues. Continuous capillaries are characterized by a complete endothelial lining with tight junctions between endothelial cells. Although a tight junction is usually impermeable and only allows for the passage of water and ions, they are often incomplete in capillaries, leaving intercellular clefts that allow for exchange of water and other very small molecules between the blood plasma and the interstitial fluid. Substances that can pass between cells include metabolic products, such as glucose, water, and small hydrophobic molecules like gases and hormones, as well as various leukocytes. Continuous capillaries not associated with the brain are rich in transport vesicles, contributing to either endocytosis or exocytosis. Those in the brain are part of the blood-brain barrier. Here, there are tight junctions and no intercellular clefts, plus a thick basement membrane and astrocyte extensions called end feet; these structures combine to prevent the movement of nearly all substances.
Figure 20.5 Types of Capillaries The three major types of capillaries: continuous, fenestrated, and sinusoid.
Fenestrated Capillaries
A fenestrated capillary is one that has pores (or fenestrations) in addition to tight junctions in the endothelial lining. These make the capillary permeable to larger molecules. The number of fenestrations and their degree of permeability vary, however, according to their location. Fenestrated capillaries are common in the small intestine, which is the primary site of nutrient absorption, as well as in the kidneys, which filter the blood. They are also found in the choroid plexus of the brain and many endocrine structures, including the hypothalamus, pituitary, pineal, and thyroid glands.
Sinusoid Capillaries
A sinusoid capillary (or sinusoid) is the least common type of capillary. Sinusoid capillaries are flattened, and they have extensive intercellular gaps and incomplete basement membranes, in addition to intercellular clefts and fenestrations. This gives them an appearance not unlike Swiss cheese. These very large openings allow for the passage of the largest molecules, including plasma proteins and even cells. Blood flow through sinusoids is very slow, allowing more time for exchange of gases, nutrients, and wastes. Sinusoids are found in the liver and spleen, bone marrow, lymph nodes (where they carry lymph, not blood), and many endocrine glands including the pituitary and adrenal glands. Without these specialized capillaries, these organs would not be able to provide their myriad of functions. For example, when bone marrow forms new blood cells, the cells must enter the blood supply and can only do so through the large openings of a sinusoid capillary; they cannot pass through the small openings of continuous or fenestrated capillaries. The liver also requires extensive specialized sinusoid capillaries in order to process the materials brought to it by the hepatic portal vein from both the digestive tract and spleen, and to release plasma proteins into circulation.
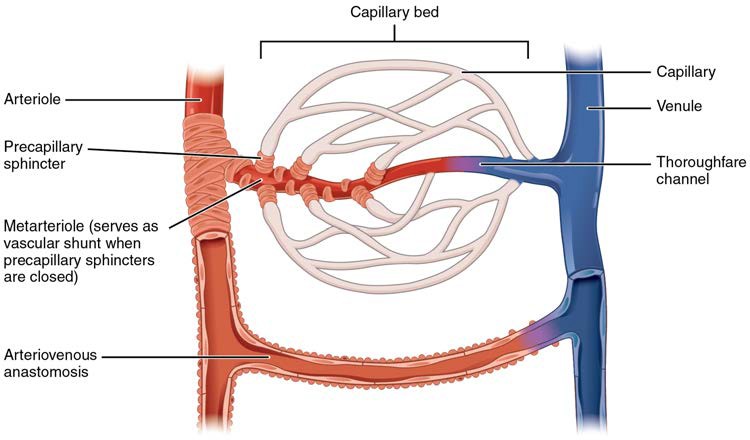
[20.1.5] Metarterioles and Capillary Beds
A metarteriole is a type of vessel that has structural characteristics of both an arteriole and a capillary. Slightly larger than the typical capillary, the smooth muscle of the tunica media of the metarteriole is not continuous but forms rings of smooth muscle (sphincters) prior to the entrance to the capillaries. Each metarteriole arises from a terminal arteriole and branches to supply blood to a capillary bed that may consist of 10–100 capillaries.
The precapillary sphincters, circular smooth muscle cells that surround the capillary at its origin with the metarteriole, tightly regulate the flow of blood from a metarteriole to the capillaries it supplies. Their function is critical: If all of the capillary beds in the body were to open simultaneously, they would collectively hold every drop of blood in the body and there would be none in the arteries, arterioles, venules, veins, or the heart itself. Normally, the precapillary sphincters are closed. When the surrounding tissues need oxygen and have excess waste products, the precapillary sphincters open, allowing blood to flow through and exchange to occur before closing once more (Figure 20.6). If all of the precapillary sphincters in a capillary bed are closed, blood will flow from the metarteriole directly into a thoroughfare channel and then into the venous circulation, bypassing the capillary bed entirely. This creates what is known as a vascular shunt. In addition, an arteriovenous anastomosis may bypass the capillary bed and lead directly to the venous system.
Although you might expect blood flow through a capillary bed to be smooth, in reality, it moves with an irregular, pulsating flow. This pattern is called vasomotion and is regulated by chemical signals that are triggered in response to changes in internal conditions, such as oxygen, carbon dioxide, hydrogen ion, and lactic acid levels. For example, during strenuous exercise when oxygen levels decrease and carbon dioxide, hydrogen ion, and lactic acid levels all increase, the capillary beds in skeletal muscle are open, as they would be in the digestive system when nutrients are present in the digestive tract. During sleep or rest periods, vessels in both areas are largely closed; they open only occasionally to allow oxygen and nutrient supplies to travel to the tissues to maintain basic life processes.
Figure 20.6 Capillary Bed In a capillary bed, arterioles give rise to metarterioles. Precapillary sphincters located at the junction of a metarteriole with a capillary regulate blood flow. A thoroughfare channel connects the metarteriole to a venule. An arteriovenous anastomosis, which directly connects the arteriole with the venule, is shown at the bottom.
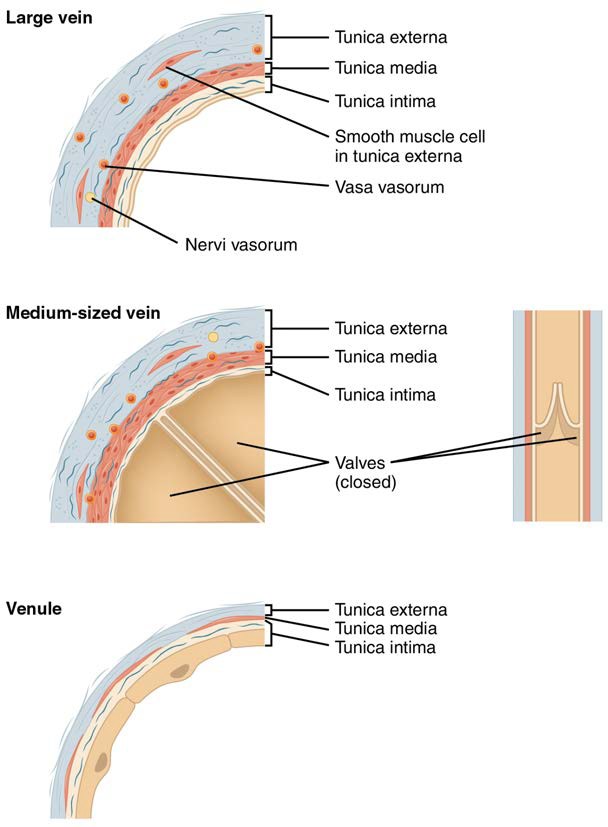
[20.1.6] Venules
A venule is an extremely small vein, generally 8–100 micrometers in diameter. Postcapillary venules join multiple capillaries exiting from a capillary bed. Multiple venules join to form veins. The walls of venules consist of endothelium, a thin middle layer with a few muscle cells and elastic fibers, plus an outer layer of connective tissue fibers that constitute a very thin tunica externa (Figure 20.7). Venules as well as capillaries are the primary sites of emigration or diapedesis, in which the white blood cells adhere to the endothelial lining of the vessels and then squeeze through adjacent cells to enter the tissue fluid.
Veins
A vein is a blood vessel that conducts blood toward the heart. Compared to arteries, veins are thin-walled vessels with large and irregular lumens (see Figure 20.7). Because they are low-pressure vessels, larger veins are commonly equipped with valves that promote the unidirectional flow of blood toward the heart and prevent backflow toward the capillaries caused by the inherent low blood pressure in veins as well as the pull of gravity. Table 20.2 compares the features of arteries and veins.
Figure 20.7 Comparison of Veins and Venules Many veins have valves to prevent back flow of blood, whereas venules do not. In terms of scale, the diameter of a venule is measured in micrometers compared to millimeters for veins.
Comparison of Arteries and Veins
| Arteries | Veins | |
| Direction of blood flow | Conducts blood away from the heart | Conducts blood toward the heart |
| General appearance | Rounded | Irregular, often collapsed |
| Pressure | High | Low |
| Wall thickness | Thick | Thin |
| Relative oxygen concentration | Higher in systemic arteries. Lower in pulmonary arteries | Lower in systemic veins. Higher in pulmonary veins |
| Valves | Not present | Present most commonly in limbs and in veins inferior to the heart |
Table 20.2
Clinical Connection: Edema and Varicose Veins
Despite the presence of valves and the contributions of other anatomical and physiological adaptations we will cover shortly, over the course of a day, some blood will inevitably pool, especially in the lower limbs, due to the pull of gravity. Any blood that accumulates in a vein will increase the pressure within it, which can then be reflected back into the smaller veins, venules, and eventually even the capillaries. Increased pressure will promote the flow of fluids out of the capillaries and into the interstitial fluid. The presence of excess tissue fluid around the cells leads to a condition called edema.
Most people experience a daily accumulation of tissue fluid, especially if they spend much of their work life on their feet (like most health professionals). However, clinical edema goes beyond normal swelling and requires medical treatment. Edema has many potential causes, including hypertension and heart failure, severe protein deficiency, renal failure, and many others. In order to treat edema, which is a sign rather than a discrete disorder, the underlying cause must be diagnosed and alleviated.
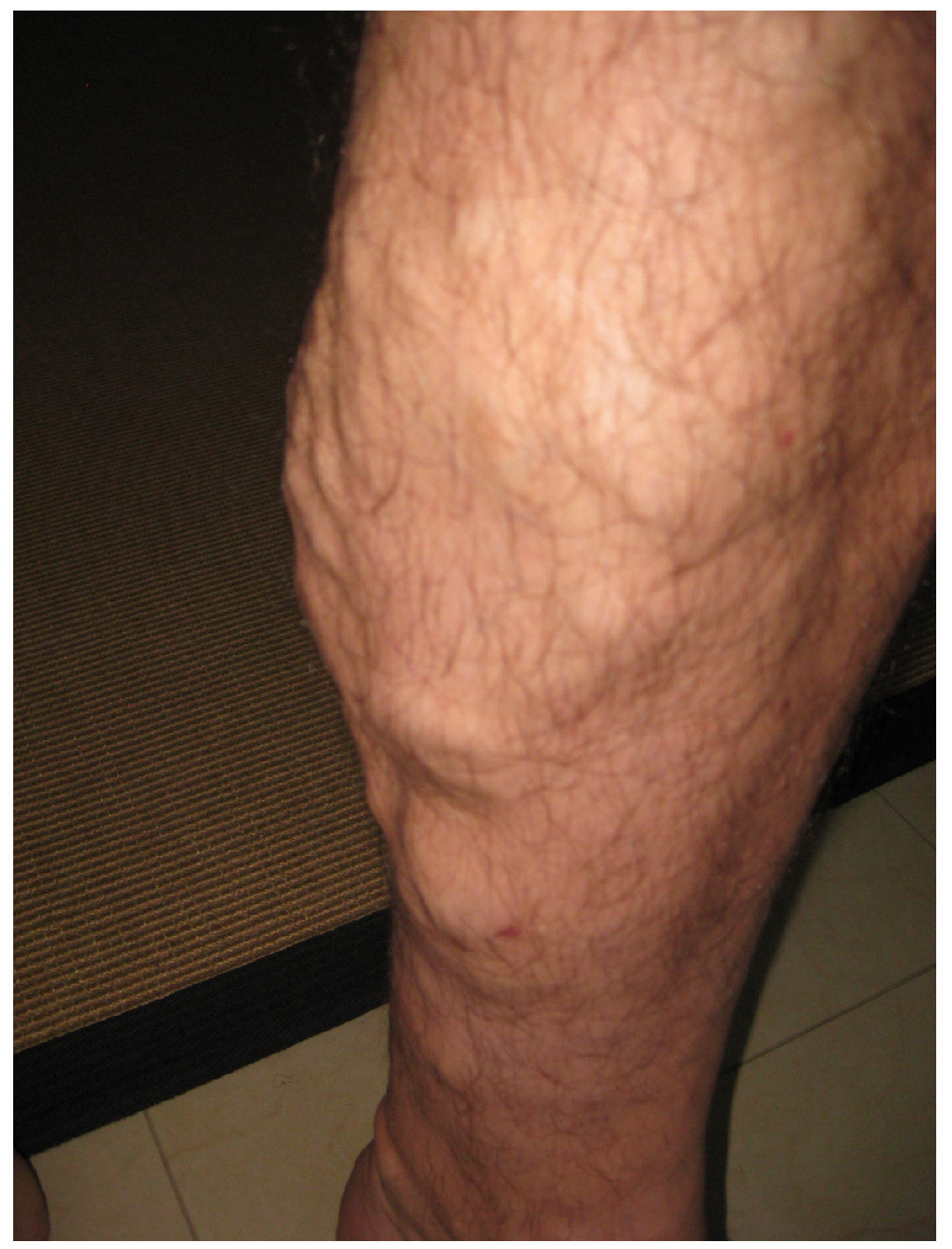
Figure 20.8 Varicose Veins Varicose veins are commonly found in the lower limbs. (credit: Thomas Kriese)
Edema may be accompanied by varicose veins, especially in the superficial veins of the legs (Figure 20.8). This disorder arises when defective valves allow blood to accumulate within the veins, causing them to distend, twist, and become visible on the surface of the integument. Varicose veins may occur in both sexes, but are more common in women and are often related to pregnancy. More than simple cosmetic blemishes, varicose veins are often painful and sometimes itchy or throbbing. Without treatment, they tend to grow worse over time. The use of support hose, as well as elevating the feet and legs whenever possible, may be helpful in alleviating this condition. Laser surgery and interventional radiologic procedures can reduce the size and severity of varicose veins. Severe cases may require conventional surgery to remove the damaged vessels. As there are typically redundant circulation patterns, that is, anastomoses, for the smaller and more superficial veins, removal does not typically impair the circulation. There is evidence that patients with varicose veins suffer a greater risk of developing a thrombus or clot.
Veins as Blood Reservoirs
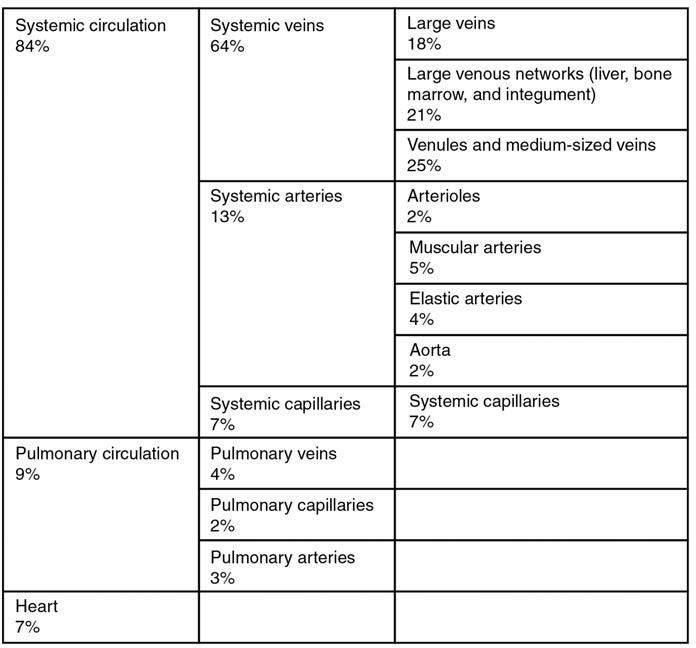
In addition to their primary function of returning blood to the heart, veins may be considered blood reservoirs, since systemic veins contain approximately 64% of the blood volume at any given time (Figure 20.9). Their ability to hold this much blood is due to their high capacitance, that is, their capacity to distend (expand) readily to store a high volume of blood, even at a low pressure. The large lumens and relatively thin walls of veins make them far more distensible than arteries; thus, they are said to be capacitance vessels.
Figure 20.9 Distribution of Blood Flow
When blood flow needs to be redistributed to other portions of the body, the vasomotor center located in the medulla oblongata sends sympathetic stimulation to the smooth muscles in the walls of the veins, causing constriction—or in this case, venoconstriction. Less dramatic than the vasoconstriction seen in smaller arteries and arterioles, venoconstriction may be likened to a “stiffening” of the vessel wall. This increases pressure on the blood within the veins, speeding its return to the heart. As you will note in Figure 20.9, approximately 21% of the venous blood is located in venous networks within the liver, bone marrow, and integument. This volume of blood is referred to as venous reserve. Through venoconstriction, this “reserve” volume of blood can get back to the heart more quickly for redistribution to other parts of the circulation.
[20.2] Blood Flow, Blood Pressure, and Resistance
Learning Objectives
By the end of this section, you will be able to:
- Distinguish between systolic pressure, diastolic pressure, pulse pressure, and mean arterial pressure
- Describe the clinical measurement of pulse and blood pressure
- Identify and discuss five variables affecting arterial blood flow and blood pressure
- Discuss several factors affecting blood flow in the venous system
Blood flow refers to the movement of blood through a vessel, tissue, or organ, and is usually expressed in terms of volume of blood per unit of time. It is initiated by the contraction of the ventricles of the heart. Ventricular contraction ejects blood into the major arteries, resulting in flow from regions of higher pressure to regions of lower pressure, as blood encounters smaller arteries and arterioles, then capillaries, then the venules and veins of the venous system. This section discusses a number of critical variables that contribute to blood flow throughout the body. It also discusses the factors that impede or slow blood flow, a phenomenon known as resistance.
As noted earlier, hydrostatic pressure is the force exerted by a fluid due to gravitational pull, usually against the wall of the container in which it is located. One form of hydrostatic pressure is blood pressure, the force exerted by blood upon the walls of the blood vessels or the chambers of the heart. Blood pressure may be measured in capillaries and veins, as well as the vessels of the pulmonary circulation; however, the term blood pressure without any specific descriptors typically refers to systemic arterial blood pressure—that is, the pressure of blood flowing in the arteries of the systemic circulation. In clinical practice, this pressure is measured in millimeters of mercury (mm Hg) and is usually obtained using the brachial artery of the arm.
[20.2.1] Components of Arterial Blood Pressure
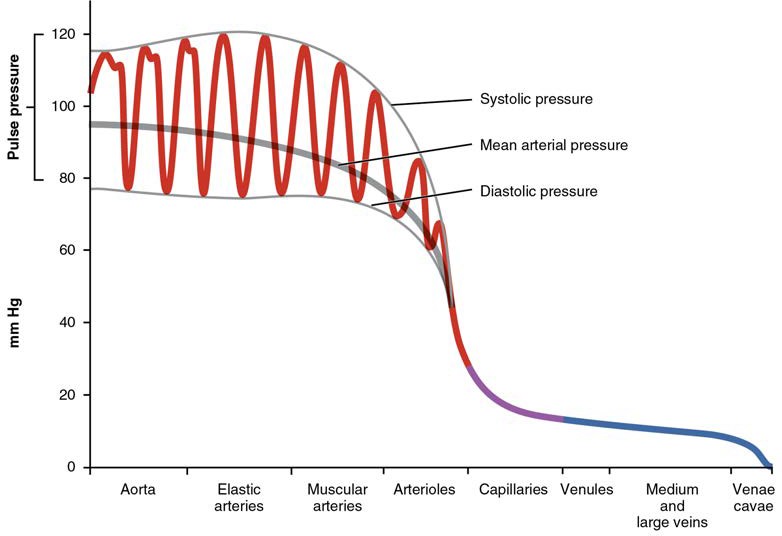
Arterial blood pressure in the larger vessels consists of several distinct components (Figure 20.10): systolic and diastolic pressures, pulse pressure, and mean arterial pressure.
Systolic and Diastolic Pressures
When systemic arterial blood pressure is measured, it is recorded as a ratio of two numbers (e.g., 120/80 mm Hg is a normal adult blood pressure), expressed as systolic pressure over diastolic pressure. The systolic pressure is the higher value (typically around 120 mm Hg) and reflects the arterial pressure resulting from the ejection of blood during ventricular contraction, or systole. The diastolic pressure is the lower value (usually about 80 mm Hg) and represents the arterial pressure of blood during ventricular relaxation, or diastole.
Figure 20.10 Systemic Blood Pressure The graph shows the components of blood pressure throughout the blood vessels, including systolic, diastolic, mean arterial, and pulse pressures.
[20.2.2] Pulse Pressure
As shown in Figure 20.10, the difference between the systolic pressure and the diastolic pressure is the pulse pressure. For example, an individual with a systolic pressure of 120 mm Hg and a diastolic pressure of 80 mm Hg would have a pulse pressure of 40 mm Hg.
Generally, a pulse pressure should be at least 25% of the systolic pressure. A pulse pressure below this level is described as low or narrow. This may occur, for example, in patients with a low stroke volume, which may be seen in congestive heart failure, stenosis of the aortic semilunar valve, or significant blood loss following trauma. In contrast, a high or wide pulse pressure is common in healthy people following strenuous exercise, when their resting pulse pressure of 30–40 mm Hg may increase temporarily to 100 mm Hg as stroke volume increases. A persistently high pulse pressure at or above 100 mm Hg may indicate excessive resistance in the arteries and can be caused by a variety of disorders. Chronic high resting pulse pressures can degrade the heart, brain, and kidneys, and warrant medical treatment.
Mean Arterial Pressure
Mean arterial pressure (MAP) represents the “average” pressure of blood in the arteries, that is, the average force driving blood into vessels that serve the tissues. Mean is a statistical concept and is calculated by taking the sum of the values divided by the number of values. Although complicated to measure directly and complicated to calculate, MAP can be approximated by adding the diastolic pressure to one-third of the pulse pressure or systolic pressure minus the diastolic pressure:
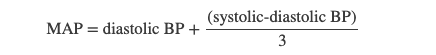
In Figure 20.10, this value is approximately 80 + (120 − 80) / 3, or 93.33. Normally, the MAP falls within the range of 70–110 mm Hg. If the value falls below 60 mm Hg for an extended time, blood pressure will not be high enough to ensure circulation to and through the tissues, which results in ischemia, or insufficient blood flow. A condition called hypoxia, inadequate oxygenation of tissues, commonly accompanies ischemia. The term hypoxemia refers to low levels of oxygen in systemic arterial blood. Neurons are especially sensitive to hypoxia and may die or be damaged if blood flow and oxygen supplies are not quickly restored.
[20.2.3] Pulse
After blood is ejected from the heart, elastic fibers in the arteries help maintain a high-pressure gradient as they expand to accommodate the blood, then recoil. This expansion and recoiling effect, known as the pulse, can be palpated manually or measured electronically. Although the effect diminishes over distance from the heart, elements of the systolic and diastolic components of the pulse are still evident down to the level of the arterioles.
Because pulse indicates heart rate, it is measured clinically to provide clues to a patient’s state of health. It is recorded as beats per minute. Both the rate and the strength of the pulse are important clinically. A high or irregular pulse rate can be caused by physical activity or other temporary factors, but it may also indicate a heart condition. The pulse strength indicates the strength of ventricular contraction and cardiac output. If the pulse is strong, then systolic pressure is high. If it is weak, systolic pressure has fallen, and medical intervention may be warranted.
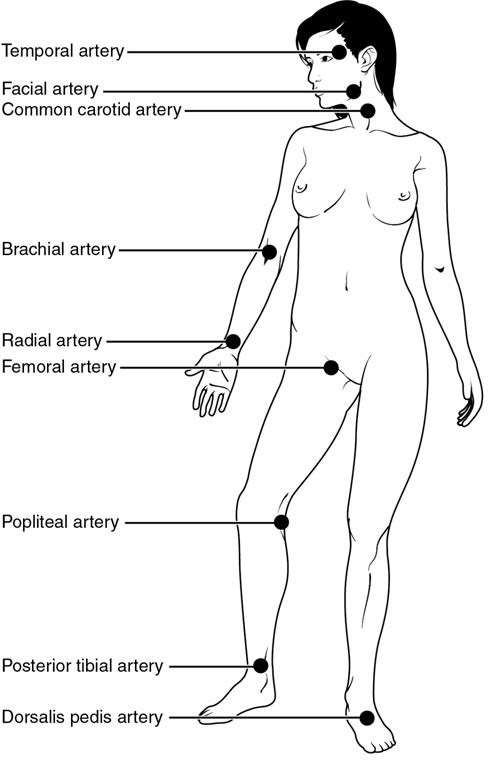
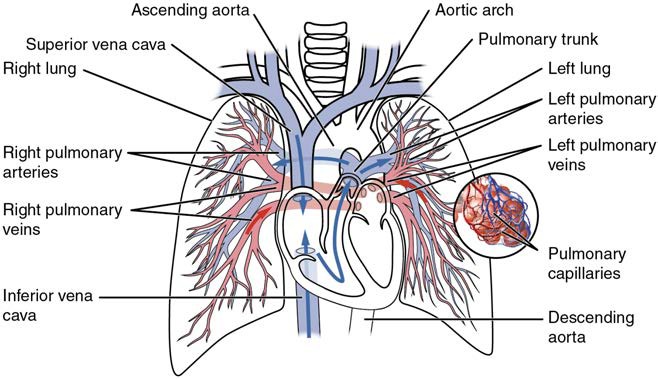
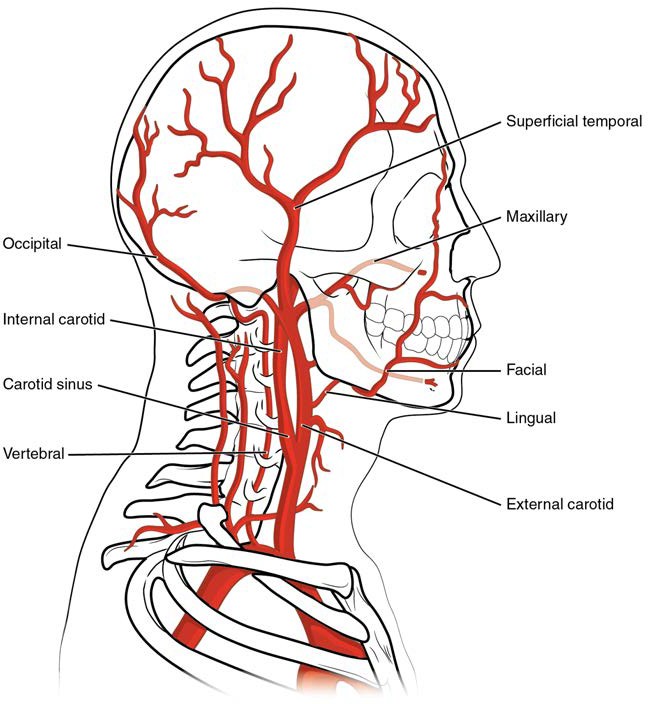
Pulse can be palpated manually by placing the tips of the fingers across an artery that runs close to the body surface and pressing lightly. While this procedure is normally performed using the radial artery in the wrist or the common carotid artery in the neck, any superficial artery that can be palpated may be used (Figure 20.11). Common sites to find a pulse include temporal and facial arteries in the head, brachial arteries in the upper arm, femoral arteries in the thigh, popliteal arteries behind the knees, posterior tibial arteries near the medial tarsal regions, and dorsalis pedis arteries in the feet. A variety of commercial electronic devices are also available to measure pulse.
Figure 20.11 Pulse Sites The pulse is most readily measured at the radial artery, but can be measured at any of the pulse points shown.
[20.2.4] Measurement of Blood Pressure
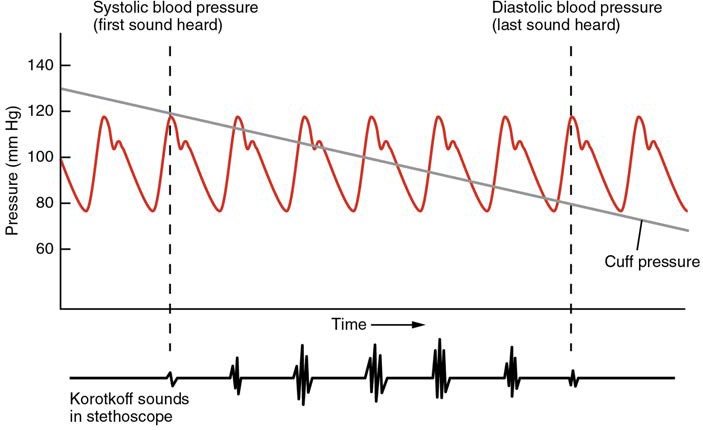
Blood pressure is one of the critical parameters measured on virtually every patient in every healthcare setting. The technique used today was developed more than 100 years ago by a pioneering Russian physician, Dr. Nikolai Korotkoff. Turbulent blood flow through the vessels can be heard as a soft ticking while measuring blood pressure; these sounds are known as Korotkoff sounds. The technique of measuring blood pressure requires the use of a sphygmomanometer (a blood pressure cuff attached to a measuring device) and a stethoscope. The technique is as follows:
- The clinician wraps an inflatable cuff tightly around the patient’s arm at about the level of the heart.
- The clinician squeezes a rubber pump to inject air into the cuff, raising pressure around the artery and temporarily cutting off blood flow into the patient’s arm.
- The clinician places the stethoscope on the patient’s antecubital region and, while gradually allowing air within the cuff to escape, listens for the Korotkoff sounds.
Although there are five recognized Korotkoff sounds, only two are normally recorded. Initially, no sounds are heard since there is no blood flow through the vessels, but as air pressure drops, the cuff relaxes, and blood flow returns to the arm. As shown in Figure 20.12, the first sound heard through the stethoscope—the first Korotkoff sound—indicates systolic pressure. As more air is released from the cuff, blood is able to flow freely through the brachial artery and all sounds disappear. The point at which the last sound is heard is recorded as the patient’s diastolic pressure.
Figure 20.12 Blood Pressure Measurement When pressure in a sphygmomanometer cuff is released, a clinician can hear the Korotkoff sounds. In this graph, a blood pressure tracing is aligned to a measurement of systolic and diastolic pressures.
The majority of hospitals and clinics have automated equipment for measuring blood pressure that work on the same principles. An even more recent innovation is a small instrument that wraps around a patient’s wrist. The patient then holds the wrist over the heart while the device measures blood flow and records pressure.
[20.2.5] Variables Affecting Blood Flow and Blood Pressure
Five variables influence blood flow and blood pressure:
- Cardiac output
- Compliance
- Volume of the blood
- Viscosity of the blood
- Blood vessel length and diameter
Recall that blood moves from higher pressure to lower pressure. It is pumped from the heart into the arteries at high pressure. If you increase pressure in the arteries (afterload), and cardiac function does not compensate, blood flow will actually decrease. In the venous system, the opposite relationship is true. Increased pressure in the veins does not decrease flow as it does in arteries, but actually increases flow. Since pressure in the veins is normally relatively low, for blood to flow back into the heart, the pressure in the atria during atrial diastole must be even lower. It normally approaches zero, except when the atria contract (see Figure 20.10).
Cardiac Output
Cardiac output is the measurement of blood flow from the heart through the ventricles, and is usually measured in liters per minute. Any factor that causes cardiac output to increase, by elevating heart rate or stroke volume or both, will elevate blood pressure and promote blood flow. These factors include sympathetic stimulation, the catecholamines epinephrine and norepinephrine, thyroid hormones, and increased calcium ion levels. Conversely, any factor that decreases cardiac output, by decreasing heart rate or stroke volume or both, will decrease arterial pressure and blood flow. These factors include parasympathetic stimulation, elevated or decreased potassium ion levels, decreased calcium levels, anoxia, and acidosis.
Compliance
Compliance is the ability of any compartment to expand to accommodate increased content. A metal pipe, for example, is not compliant, whereas a balloon is. The greater the compliance of an artery, the more effectively it is able to expand to accommodate surges in blood flow without increased resistance or blood pressure. Veins are more compliant than arteries and can expand to hold more blood. When vascular disease causes stiffening of arteries, compliance is reduced and resistance to blood flow is increased. The result is more turbulence, higher pressure within the vessel, and reduced blood flow. This increases the work of the heart.
A Mathematical Approach to Factors Affecting Blood Flow
Jean Louis Marie Poiseuille was a French physician and physiologist who devised a mathematical equation describing blood flow and its relationship to known parameters. The same equation also applies to engineering studies of the flow of fluids. Although understanding the math behind the relationships among the factors affecting blood flow is not necessary to understand blood flow, it can help solidify an understanding of their relationships. Please note that even if the equation looks intimidating, breaking it down into its components and following the relationships will make these relationships clearer, even if you are weak in math. Focus on the three critical variables: radius (r), vessel length (λ), and viscosity (η).
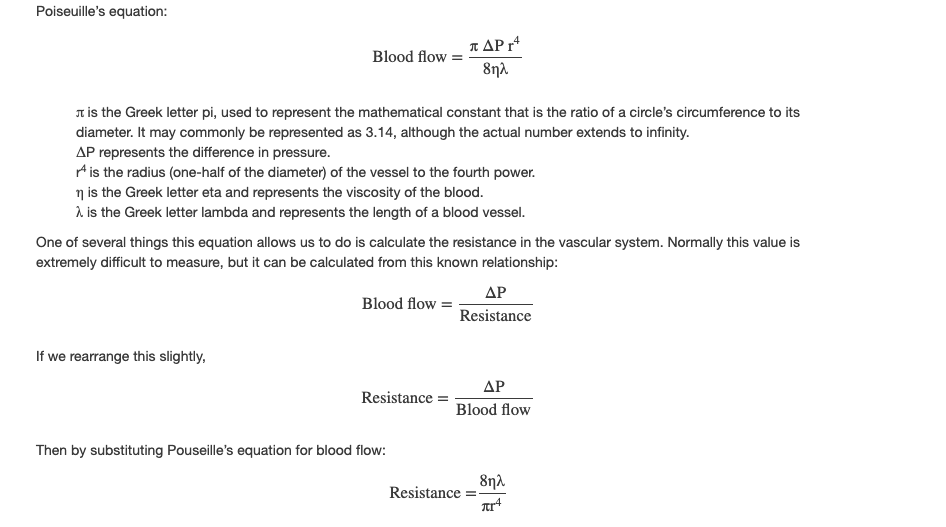
By examining this equation, you can see that there are only three variables: viscosity, vessel length, and radius, since 8 and π are both constants. The important thing to remember is this: Two of these variables, viscosity and vessel length, will change slowly in the body. Only one of these factors, the radius, can be changed rapidly by vasoconstriction and vasodilation, thus dramatically impacting resistance and flow. Further, small changes in the radius will greatly affect flow, since it is raised to the fourth power in the equation.
We have briefly considered how cardiac output and blood volume impact blood flow and pressure; the next step is to see how the other variables (contraction, vessel length, and viscosity) articulate with Pouseille’s equation and what they can teach us about the impact on blood flow.
Blood Volume
The relationship between blood volume, blood pressure, and blood flow is intuitively obvious. Water may merely trickle along a creek bed in a dry season, but rush quickly and under great pressure after a heavy rain. Similarly, as blood volume decreases, pressure and flow decrease. As blood volume increases, pressure and flow increase.
Under normal circumstances, blood volume varies little. Low blood volume, called hypovolemia, may be caused by bleeding, dehydration, vomiting, severe burns, or some medications used to treat hypertension. It is important to recognize that other regulatory mechanisms in the body are so effective at maintaining blood pressure that an individual may be asymptomatic until 10–20% of the blood volume has been lost. Treatment typically includes intravenous fluid replacement.
Hypervolemia, excessive fluid volume, may be caused by retention of water and sodium, as seen in patients with heart failure, liver cirrhosis, some forms of kidney disease, hyperaldosteronism, and some glucocorticoid steroid treatments. Restoring homeostasis in these patients depends upon reversing the condition that triggered the hypervolemia.
Blood Viscosity
Viscosity is the thickness of fluids that affects their ability to flow. Clean water, for example, is less viscous than mud. The viscosity of blood is directly proportional to resistance and inversely proportional to flow; therefore, any condition that causes viscosity to increase will also increase resistance and decrease flow. For example, imagine sipping milk, then a milkshake, through the same size straw. You experience more resistance and therefore less flow from the milkshake. Conversely, any condition that causes viscosity to decrease (such as when the milkshake melts) will decrease resistance and increase flow.
Normally the viscosity of blood does not change over short periods of time. The two primary determinants of blood viscosity are the formed elements and plasma proteins. Since the vast majority of formed elements are erythrocytes, any condition affecting erythropoiesis, such as polycythemia or anemia, can alter viscosity. Since most plasma proteins are produced by the liver, any condition affecting liver function can also change the viscosity slightly and therefore alter blood flow. Liver abnormalities such as hepatitis, cirrhosis, alcohol damage, and drug toxicities result in decreased levels of plasma proteins, which decrease blood viscosity. While leukocytes and platelets are normally a small component of the formed elements, there are some rare conditions in which severe overproduction can impact viscosity as well.
Vessel Length and Diameter
The length of a vessel is directly proportional to its resistance: the longer the vessel, the greater the resistance and the lower the flow. As with blood volume, this makes intuitive sense, since the increased surface area of the vessel will impede the flow of blood. Likewise, if the vessel is shortened, the resistance will decrease and flow will increase.
The length of our blood vessels increases throughout childhood as we grow, of course, but is unchanging in adults under normal physiological circumstances. Further, the distribution of vessels is not the same in all tissues. Adipose tissue has around half the vascular supply of skeletal muscle. Overall, vessels decrease in length only during loss of mass or amputation.
In contrast to length, the diameter of blood vessels changes throughout the body, according to the type of vessel, as we discussed earlier. The diameter of any given vessel may also change frequently throughout the day in response to neural and chemical signals that trigger vasodilation and vasoconstriction. The vascular tone of the vessel is the contractile state of the smooth muscle and the primary determinant of diameter, and thus of resistance and flow. The effect of vessel diameter on resistance is inverse: Given the same volume of blood, an increased diameter means there is less blood contacting the vessel wall, thus lower friction and lower resistance, subsequently increasing flow. A decreased diameter means more of the blood contacts the vessel wall, and resistance increases, subsequently decreasing flow.
The influence of lumen diameter on resistance is dramatic: A slight increase or decrease in diameter causes a huge decrease or increase in resistance. This is because resistance is inversely proportional to the radius of the blood vessel (one-half of the vessel’s diameter) raised to the fourth power (R = 1/r4). This means, for example, that if an artery or arteriole constricts to one-half of its original radius, the resistance to flow will increase 16 times. And if an artery or arteriole dilates to twice its initial radius, then resistance in the vessel will decrease to 1/16 of its original value and flow will increase 16 times.
The Roles of Vessel Diameter and Total Area in Blood Flow and Blood Pressure
Recall that we classified arterioles as resistance vessels, because given their small lumen, they dramatically slow the flow of blood from arteries. In fact, arterioles are the site of greatest resistance in the entire vascular network. This may seem surprising, given that capillaries have a smaller size. How can this phenomenon be explained?
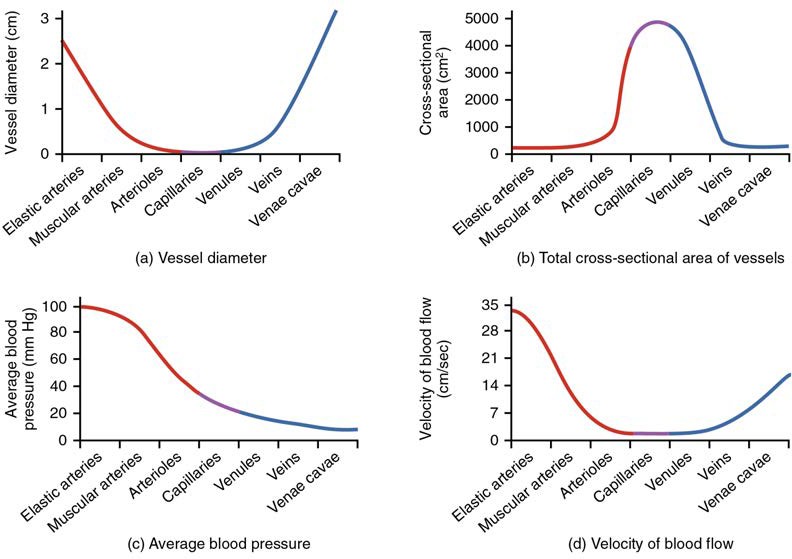
Figure 20.13 compares vessel diameter, total cross-sectional area, average blood pressure, and blood velocity through the systemic vessels. Notice in parts (a) and (b) that the total cross-sectional area of the body’s capillary beds is far greater than any other type of vessel. Although the diameter of an individual capillary is significantly smaller than the diameter of an arteriole, there are vastly more capillaries in the body than there are other types of blood vessels. Part (c) shows that blood pressure drops unevenly as blood travels from arteries to arterioles, capillaries, venules, and veins, and encounters greater resistance. However, the site of the most precipitous drop, and the site of greatest resistance, is the arterioles. This explains why vasodilation and vasoconstriction of arterioles play more significant roles in regulating blood pressure than do the vasodilation and vasoconstriction of other vessels.
Part (d) shows that the velocity (speed) of blood flow decreases dramatically as the blood moves from arteries to arterioles to capillaries. This slow flow rate allows more time for exchange processes to occur. As blood flows through the veins, the rate of velocity increases, as blood is returned to the heart.
Figure 20.13 Relationships among Vessels in the Systemic Circuit The relationships among blood vessels that can be compared include (a) vessel diameter, (b) total cross-sectional area, (c) average blood pressure, and (d) velocity of blood flow.
Cardiovascular System: Arteriosclerosis
Compliance allows an artery to expand when blood is pumped through it from the heart, and then to recoil after the surge has passed. This helps promote blood flow. In arteriosclerosis, compliance is reduced, and pressure and resistance within the vessel increase. This is a leading cause of hypertension and coronary heart disease, as it causes the heart to work harder to generate a pressure great enough to overcome the resistance.
Arteriosclerosis begins with injury to the endothelium of an artery, which may be caused by irritation from high blood glucose, infection, tobacco use, excessive blood lipids, and other factors. Artery walls that are constantly stressed by blood flowing at high pressure are also more likely to be injured—which means that hypertension can promote arteriosclerosis, as well as result from it.
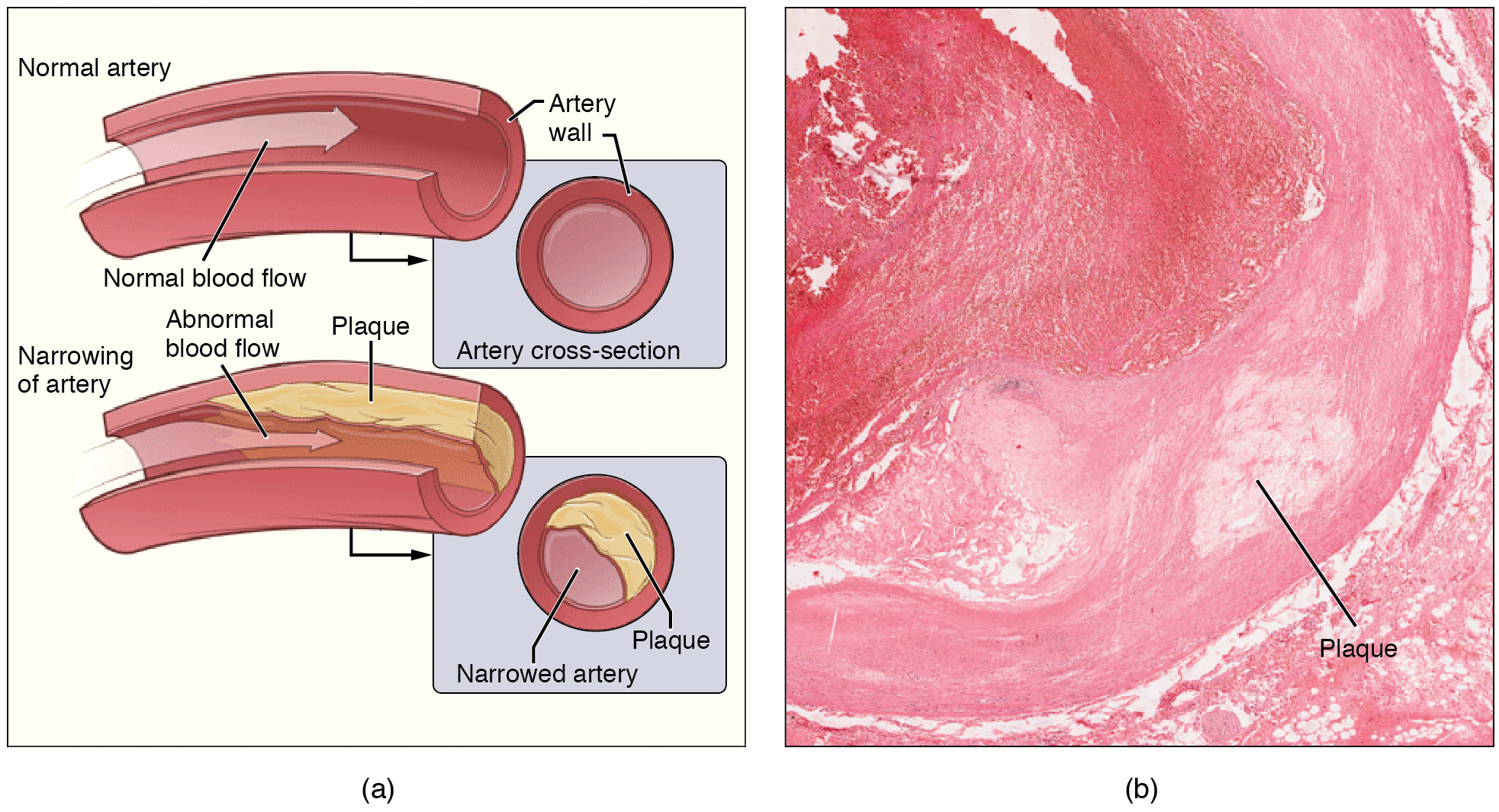
Recall that tissue injury causes inflammation. As inflammation spreads into the artery wall, it weakens and scars it, leaving it stiff (sclerotic). As a result, compliance is reduced. Moreover, circulating triglycerides and cholesterol can seep between the damaged lining cells and become trapped within the artery wall, where they are frequently joined by leukocytes, calcium, and cellular debris. Eventually, this buildup, called plaque, can narrow arteries enough to impair blood flow. The term for this condition, atherosclerosis (athero- = “porridge”) describes the mealy deposits (Figure 20.14).
Figure 20.14 Atherosclerosis (a) Atherosclerosis can result from plaques formed by the buildup of fatty, calcified deposits in an artery. (b) Plaques can also take other forms, as shown in this micrograph of a coronary artery that has a buildup of connective tissue within the artery wall. LM × 40. (Micrograph provided by the Regents of University of Michigan Medical School © 2012)
Sometimes a plaque can rupture, causing microscopic tears in the artery wall that allow blood to leak into the tissue on the other side. When this happens, platelets rush to the site to clot the blood. This clot can further obstruct the artery and—if it occurs in a coronary or cerebral artery—cause a sudden heart attack or stroke. Alternatively, plaque can break off and travel through the bloodstream as an embolus until it blocks a more distant, smaller artery.
Even without total blockage, vessel narrowing leads to ischemia—reduced blood flow—to the tissue region “downstream” of the narrowed vessel. Ischemia in turn leads to hypoxia—decreased supply of oxygen to the tissues. Hypoxia involving cardiac muscle or brain tissue can lead to cell death and severe impairment of brain or heart function.
A major risk factor for both arteriosclerosis and atherosclerosis is advanced age, as the conditions tend to progress over time. Arteriosclerosis is normally defined as the more generalized loss of compliance, “hardening of the arteries,” whereas atherosclerosis is a more specific term for the build-up of plaque in the walls of the vessel and is a specific type of arteriosclerosis. There is also a distinct genetic component, and pre-existing hypertension and/or diabetes also greatly increase the risk. However, obesity, poor nutrition, lack of physical activity, and tobacco use all are major risk factors.Treatment includes lifestyle changes, such as weight loss, smoking cessation, regular exercise, and adoption of a diet low in sodium and saturated fats. Medications to reduce cholesterol and blood pressure may be prescribed. For blocked coronary arteries, surgery is warranted. In angioplasty, a catheter is inserted into the vessel at the point of narrowing, and a second catheter with a balloon-like tip is inflated to widen the opening. To prevent subsequent collapse of the vessel, a small mesh tube called a stent is often inserted. In an endarterectomy, plaque is surgically removed from the walls of a vessel. This operation is typically performed on the carotid arteries of the neck, which are a prime source of oxygenated blood for the brain. In a coronary bypass procedure, a non-vital superficial vessel from another part of the body (often the great saphenous vein) or a synthetic vessel is inserted to create a path around the blocked area of a coronary artery.
Venous System
The pumping action of the heart propels the blood into the arteries, from an area of higher pressure toward an area of lower pressure. If blood is to flow from the veins back into the heart, the pressure in the veins must be greater than the pressure in the atria of the heart. Two factors help maintain this pressure gradient between the veins and the heart. First, the pressure in the atria during diastole is very low, often approaching zero when the atria are relaxed (atrial diastole). Second, two physiologic “pumps” increase pressure in the venous system. The use of the term “pump” implies a physical device that speeds flow. These physiological pumps are less obvious.
Skeletal Muscle Pump
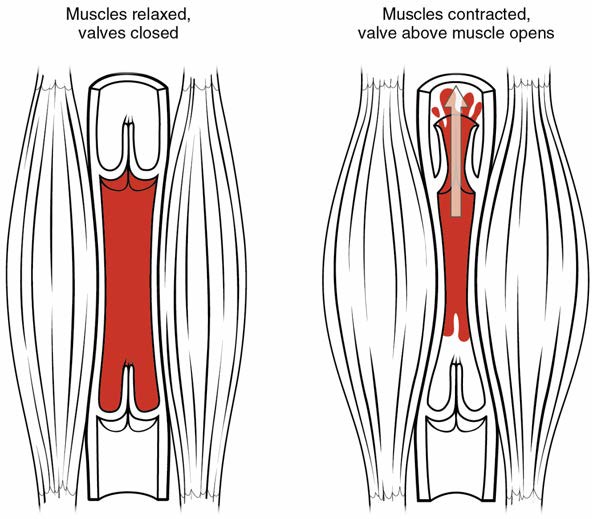
In many body regions, the pressure within the veins can be increased by the contraction of the surrounding skeletal muscle. This mechanism, known as the skeletal muscle pump (Figure 20.15), helps the lower-pressure veins counteract the force of gravity, increasing pressure to move blood back to the heart. As leg muscles contract, for example during walking or running, they exert pressure on nearby veins with their numerous one-way valves. This increased pressure causes blood to flow upward, opening valves superior to the contracting muscles so blood flows through. Simultaneously, valves inferior to the contracting muscles close; thus, blood should not seep back downward toward the feet. Military recruits are trained to flex their legs slightly while standing at attention for prolonged periods. Failure to do so may allow blood to pool in the lower limbs rather than returning to the heart. Consequently, the brain will not receive enough oxygenated blood, and the individual may lose consciousness.
Figure 20.15 Skeletal Muscle Pump The contraction of skeletal muscles surrounding a vein compresses the blood and increases the pressure in that area. This action forces blood closer to the heart where venous pressure is lower. Note the importance of the one-way valves to assure that blood flows only in the proper direction.
Respiratory Pump
The respiratory pump aids blood flow through the veins of the thorax and abdomen. During inhalation, the volume of the thorax increases, largely through the contraction of the thoracic diaphragm, which moves downward and compresses the abdominal cavity. The elevation of the chest caused by the contraction of the external intercostal muscles also contributes to the increased volume of the thorax. The volume increase causes air pressure within the thorax to decrease, allowing us to inhale. Additionally, as air pressure within the thorax drops, blood pressure in the thoracic veins also decreases, falling below the pressure in the abdominal veins. This causes blood to flow along its pressure gradient from veins outside the thorax, where pressure is higher, into the thoracic region, where pressure is now lower. This in turn promotes the return of blood from the thoracic veins to the atria. During exhalation, when air pressure increases within the thoracic cavity, pressure in the thoracic veins increases, speeding blood flow into the heart while valves in the veins prevent blood from flowing backward from the thoracic and abdominal veins.
Pressure Relationships in the Venous System
Although vessel diameter increases from the smaller venules to the larger veins and eventually to the venae cavae (singular = vena cava), the total cross-sectional area actually decreases. The individual veins are larger in diameter than the venules, but their total number is much lower, so their total cross-sectional area is also lower.
Also notice that, as blood moves from venules to veins, the average blood pressure drops, but the blood velocity actually increases. This pressure gradient drives blood back toward the heart. Again, the presence of one-way valves and the skeletal muscle and respiratory pumps contribute to this increased flow. Since approximately 64% of the total blood volume resides in systemic veins, any action that increases the flow of blood through the veins will increase venous return to the heart. Maintaining vascular tone within the veins prevents the veins from merely distending, dampening the flow of blood, and as you will see, vasoconstriction actually enhances the flow.
The Role of Venoconstriction in Resistance, Blood Pressure, and Flow
As previously discussed, vasoconstriction of an artery or arteriole decreases the radius, increasing resistance and pressure, but decreasing flow. Venoconstriction, on the other hand, has a very different outcome. The walls of veins are thin but irregular; thus, when the smooth muscle in those walls constricts, the lumen becomes more rounded. The more rounded the lumen, the less surface area the blood encounters, and the less resistance the vessel offers. Vasoconstriction increases pressure within a vein as it does in an artery, but in veins, the increased pressure increases flow. Recall that the pressure in the atria, into which the venous blood will flow, is very low, approaching zero for at least part of the relaxation phase of the cardiac cycle. Thus, venoconstriction increases the return of blood to the heart. Another way of stating this is that venoconstriction increases the preload or stretch of the cardiac muscle and increases contraction.
[20.3] Capillary Exchange
Learning Objectives
By the end of this section, you will be able to:
- Identify the primary mechanisms of capillary exchange
- Distinguish between capillary hydrostatic pressure and blood colloid osmotic pressure, explaining the contribution of each to net filtration pressure
- Compare filtration and reabsorption
- Explain the fate of fluid that is not reabsorbed from the tissues into the vascular capillaries
The primary purpose of the cardiovascular system is to circulate gases, nutrients, wastes, and other substances to and from the cells of the body. Small molecules, such as gases, lipids, and lipid-soluble molecules, can diffuse directly through the membranes of the endothelial cells of the capillary wall. Glucose, amino acids, and ions—including sodium, potassium, calcium, and chloride—use transporters to move through specific channels in the membrane by facilitated diffusion. Glucose, ions, and larger molecules may also leave the blood through intercellular clefts. Larger molecules can pass through the pores of fenestrated capillaries, and even large plasma proteins can pass through the great gaps in the sinusoids. Some large proteins in blood plasma can move into and out of the endothelial cells packaged within vesicles by endocytosis and exocytosis. Water moves by osmosis.
[20.3.1] Bulk Flow
The mass movement of fluids into and out of capillary beds requires a transport mechanism far more efficient than mere diffusion. This movement, often referred to as bulk flow, involves two pressure-driven mechanisms: volumes of fluid move from an area of higher pressure in a capillary bed to an area of lower pressure in the tissues via filtration. In contrast, the movement of fluid from an area of higher pressure in the tissues into an area of lower pressure in the capillaries is reabsorption. Two types of pressure interact to drive each of these movements: hydrostatic pressure and osmotic pressure.
Hydrostatic Pressure
The primary force driving fluid transport between the capillaries and tissues is hydrostatic pressure, which can be defined as the pressure of any fluid enclosed in a space. Blood hydrostatic pressure is the force exerted by the blood confined within blood vessels or heart chambers. Even more specifically, the pressure exerted by blood against the wall of a capillary is called capillary hydrostatic pressure (CHP), and is the same as capillary blood pressure. CHP is the force that drives fluid out of capillaries and into the tissues.
As fluid exits a capillary and moves into tissues, the hydrostatic pressure in the interstitial fluid correspondingly rises. This opposing hydrostatic pressure is called the interstitial fluid hydrostatic pressure (IFHP). Generally, the CHP originating from the arterial pathways is considerably higher than the IFHP, because lymphatic vessels are continually absorbing excess fluid from the tissues. Thus, fluid generally moves out of the capillary and into the interstitial fluid. This process is called filtration.
Osmotic Pressure
The net pressure that drives reabsorption—the movement of fluid from the interstitial fluid back into the capillaries—is called osmotic pressure (sometimes referred to as oncotic pressure). Whereas hydrostatic pressure forces fluid out of the capillary, osmotic pressure draws fluid back in. Osmotic pressure is determined by osmotic concentration gradients, that is, the difference in the solute-to-water concentrations in the blood and tissue fluid. A region higher in solute concentration (and lower in water concentration) draws water across a semipermeable membrane from a region higher in water concentration (and lower in solute concentration).
As we discuss osmotic pressure in blood and tissue fluid, it is important to recognize that the formed elements of blood do not contribute to osmotic concentration gradients. Rather, it is the plasma proteins that play the key role. Solutes also move across the capillary wall according to their concentration gradient, but overall, the concentrations should be similar and not have a significant impact on osmosis. Because of their large size and chemical structure, plasma proteins are not truly solutes, that is, they do not dissolve but are dispersed or suspended in their fluid medium, forming a colloid rather than a solution.
The pressure created by the concentration of colloidal proteins in the blood is called the blood colloidal osmotic pressure (BCOP). Its effect on capillary exchange accounts for the reabsorption of water. The plasma proteins suspended in blood cannot move across the semipermeable capillary cell membrane, and so they remain in the plasma. As a result, blood has a higher colloidal concentration and lower water concentration than tissue fluid. It therefore attracts water. We can also say that the BCOP is higher than the interstitial fluid colloidal osmotic pressure (IFCOP), which is always very low because interstitial fluid contains few proteins. Thus, water is drawn from the tissue fluid back into the capillary, carrying dissolved molecules with it. This difference in colloidal osmotic pressure accounts for reabsorption.
Interaction of Hydrostatic and Osmotic Pressures
The normal unit used to express pressures within the cardiovascular system is millimeters of mercury (mm Hg). When blood leaving an arteriole first enters a capillary bed, the CHP is quite high—about 35 mm Hg. Gradually, this initial CHP declines as the blood moves through the capillary so that by the time the blood has reached the venous end, the CHP has dropped to approximately 18 mm Hg. In comparison, the plasma proteins remain suspended in the blood, so the BCOP remains fairly constant at about 25 mm Hg throughout the length of the capillary and considerably below the osmotic pressure in the interstitial fluid.
The net filtration pressure (NFP) represents the interaction of the hydrostatic and osmotic pressures, driving fluid out of the capillary. It is equal to the difference between the CHP and the BCOP. Since filtration is, by definition, the movement of fluid out of the capillary, when reabsorption is occurring, the NFP is a negative number.
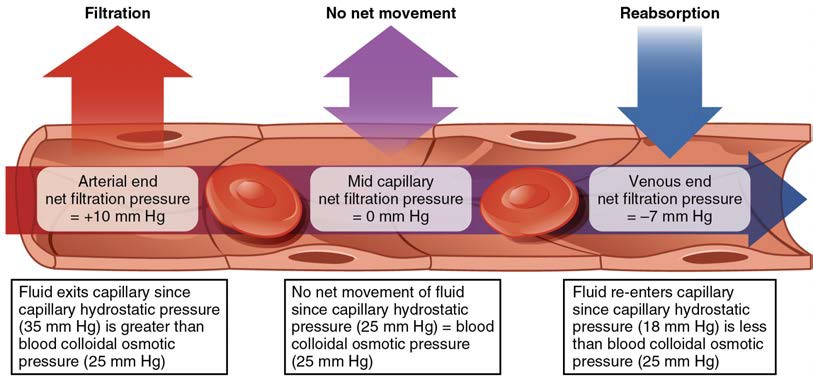
NFP changes at different points in a capillary bed (Figure 20.16). Close to the arterial end of the capillary, it is approximately 10 mm Hg, because the CHP of 35 mm Hg minus the BCOP of 25 mm Hg equals 10 mm Hg. Recall that the hydrostatic and osmotic pressures of the interstitial fluid are essentially negligible. Thus, the NFP of 10 mm Hg drives a net movement of fluid out of the capillary at the arterial end. At approximately the middle of the capillary, the CHP is about the same as the BCOP of 25 mm Hg, so the NFP drops to zero. At this point, there is no net change of volume: Fluid moves out of the capillary at the same rate as it moves into the capillary. Near the venous end of the capillary, the CHP has dwindled to about 18 mm Hg due to loss of fluid. Because the BCOP remains steady at 25 mm Hg, water is drawn into the capillary, that is, reabsorption occurs. Another way of expressing this is to say that at the venous end of the capillary, there is an NFP of −7 mm Hg.
Figure 20.16 Capillary Exchange Net filtration occurs near the arterial end of the capillary since capillary hydrostatic pressure (CHP) is greater than blood colloidal osmotic pressure (BCOP). There is no net movement of fluid near the midpoint since CHP = BCOP. Net reabsorption occurs near the venous end since BCOP is greater than CHP.
[20.3.2] The Role of Lymphatic Capillaries
Since overall CHP is higher than BCOP, it is inevitable that more net fluid will exit the capillary through filtration at the arterial end than enters through reabsorption at the venous end. Considering all capillaries over the course of a day, this can be quite a substantial amount of fluid: Approximately 24 liters per day are filtered, whereas 20.4 liters are reabsorbed. This excess fluid is picked up by capillaries of the lymphatic system. These extremely thin-walled vessels have copious numbers of valves that ensure unidirectional flow through ever-larger lymphatic vessels that eventually drain into the subclavian veins in the neck. An important function of the lymphatic system is to return the fluid (lymph) to the blood. Lymph may be thought of as recycled blood plasma.
Watch this video (http://openstaxcollege.org/l/capillaryfunct) to explore capillaries and how they function in the body. Capillaries are never more than 100 micrometers away. What is the main component of interstitial fluid?
[20.4] Homeostatic Regulation of the Vascular System
Learning Objectives
By the end of this section, you will be able to:
- Discuss the mechanisms involved in the neural regulation of vascular homeostasis
- Describe the contribution of a variety of hormones to the renal regulation of blood pressure
- Identify the effects of exercise on vascular homeostasis
- Discuss how hypertension, hemorrhage, and circulatory shock affect vascular health
In order to maintain homeostasis in the cardiovascular system and provide adequate blood to the tissues, blood flow must be redirected continually to the tissues as they become more active. In a very real sense, the cardiovascular system engages in resource allocation, because there is not enough blood flow to distribute blood equally to all tissues simultaneously. For example, when an individual is exercising, more blood will be directed to skeletal muscles, the heart, and the lungs. Following a meal, more blood is directed to the digestive system. Only the brain receives a more or less constant supply of blood whether you are active, resting, thinking, or engaged in any other activity.
Table 20.3 provides the distribution of systemic blood at rest and during exercise. Although most of the data appears logical, the values for the distribution of blood to the integument may seem surprising. During exercise, the body distributes more blood to the body surface where it can dissipate the excess heat generated by increased activity into the environment.
Systemic Blood Flow During Rest, Mild Exercise, and Maximal Exercise in a Healthy Young Individual
| Organ | Resting (mL/min) | Mild exercise (mL/min | Maximal exercise (mL/min) |
| Skeletal muscle | 1200 | 4500 | 12,500 |
| Heart | 250 | 350 | 750 |
| Brain | 750 | 750 | 750 |
| Integument | 500 | 1500 | 1900 |
| Kidney | 1100 | 900 | 600 |
| Gastrointestinal | 1400 | 1100 | 600 |
| Others (i.e., liver, spleen) | 600 | 400 | 400 |
| Total | 5800 | 9500 | 17,500 |
Table 20.3
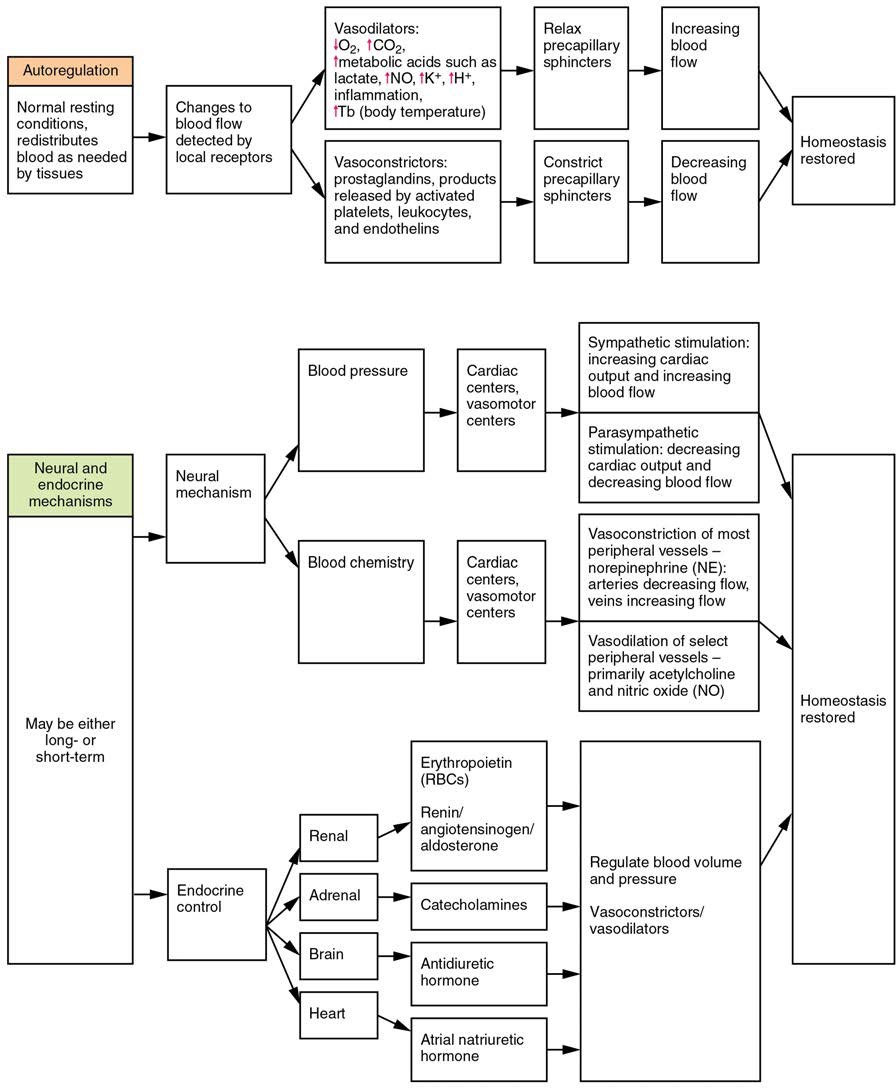
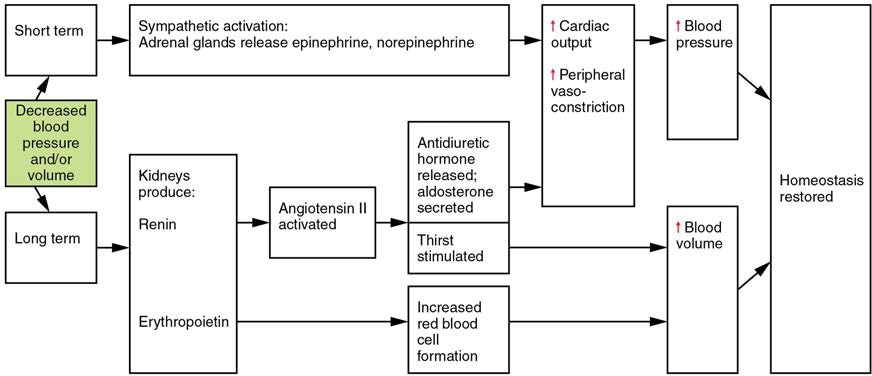
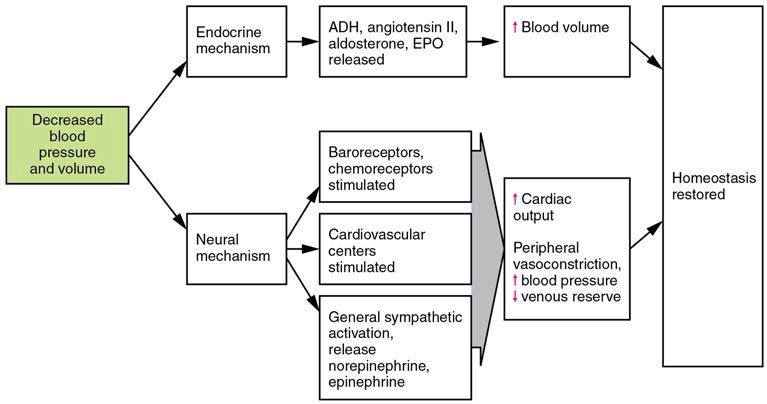
Three homeostatic mechanisms ensure adequate blood flow, blood pressure, distribution, and ultimately perfusion: neural, endocrine, and autoregulatory mechanisms. They are summarized in Figure 20.17.
Figure 20.17 Summary of Factors Maintaining Vascular Homeostasis Adequate blood flow, blood pressure, distribution, and perfusion involve autoregulatory, neural, and endocrine mechanisms.
Neural Regulation
The nervous system plays a critical role in the regulation of vascular homeostasis. The primary regulatory sites include the cardiovascular centers in the brain that control both cardiac and vascular functions. In addition, more generalized neural responses from the limbic system and the autonomic nervous system are factors.
The Cardiovascular Centers in the Brain
Neurological regulation of blood pressure and flow depends on the cardiovascular centers located in the medulla oblongata.
This cluster of neurons responds to changes in blood pressure as well as blood concentrations of oxygen, carbon dioxide, and hydrogen ions. The cardiovascular center contains three distinct paired components:
- The cardioaccelerator centers stimulate cardiac function by regulating heart rate and stroke volume via sympathetic stimulation from the cardiac accelerator nerve.
- The cardioinhibitor centers slow cardiac function by decreasing heart rate and stroke volume via parasympathetic stimulation from the vagus nerve.
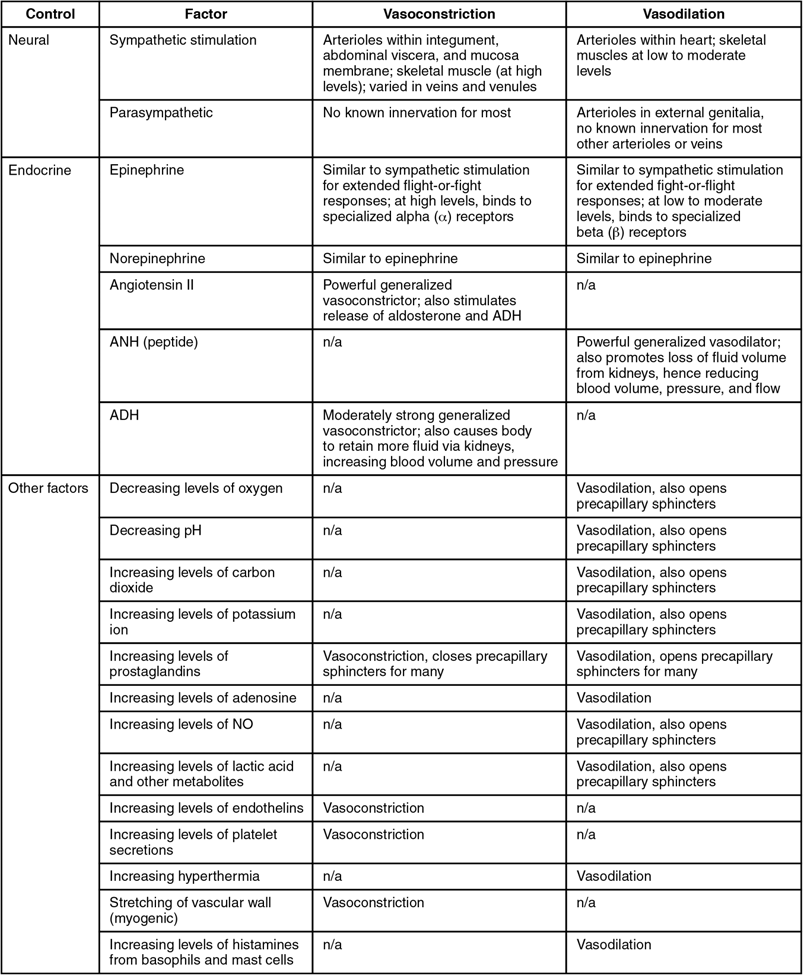
- The vasomotor centers control vessel tone or contraction of the smooth muscle in the tunica media. Changes in diameter affect peripheral resistance, pressure, and flow, which affect cardiac output. The majority of these neurons act via the release of the neurotransmitter norepinephrine from sympathetic neurons.
Although each center functions independently, they are not anatomically distinct.
There is also a small population of neurons that control vasodilation in the vessels of the brain and skeletal muscles by relaxing the smooth muscle fibers in the vessel tunics. Many of these are cholinergic neurons, that is, they release acetylcholine, which in turn stimulates the vessels’ endothelial cells to release nitric oxide (NO), which causes vasodilation. Others release norepinephrine that binds to β2 receptors. A few neurons release NO directly as a neurotransmitter.
Recall that mild stimulation of the skeletal muscles maintains muscle tone. A similar phenomenon occurs with vascular tone in vessels. As noted earlier, arterioles are normally partially constricted: With maximal stimulation, their radius may be reduced to one-half of the resting state. Full dilation of most arterioles requires that this sympathetic stimulation be suppressed. When it is, an arteriole can expand by as much as 150%. Such a significant increase can dramatically affect resistance, pressure, and flow.
Baroreceptor Reflexes
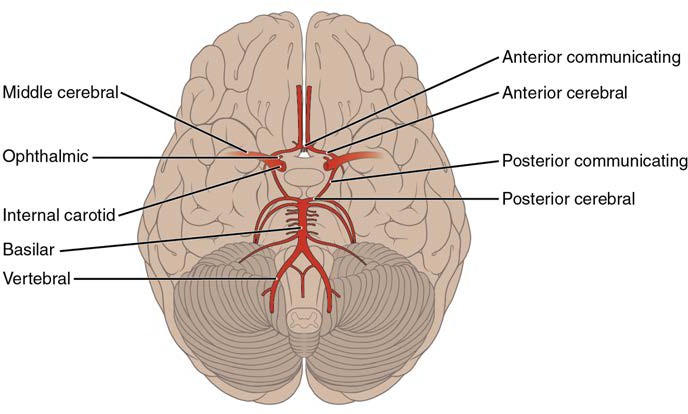
Baroreceptors are specialized stretch receptors located within thin areas of blood vessels and heart chambers that respond to the degree of stretch caused by the presence of blood. They send impulses to the cardiovascular center to regulate blood pressure. Vascular baroreceptors are found primarily in sinuses (small cavities) within the aorta and carotid arteries: The aortic sinuses are found in the walls of the ascending aorta just superior to the aortic valve, whereas the carotid sinuses are in the base of the internal carotid arteries. There are also low-pressure baroreceptors located in the walls of the venae cavae and right atrium.
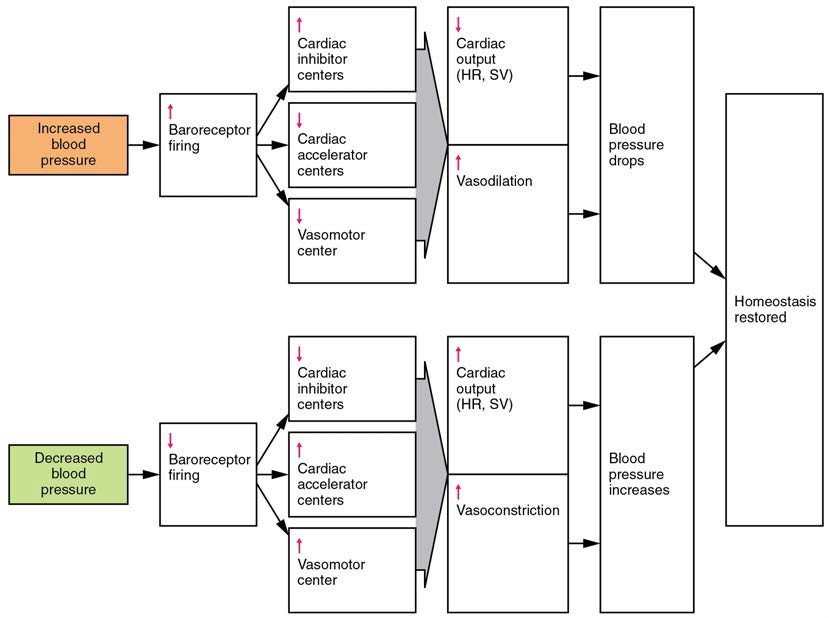
When blood pressure increases, the baroreceptors are stretched more tightly and initiate action potentials at a higher rate. At lower blood pressures, the degree of stretch is lower and the rate of firing is slower. When the cardiovascular center in the medulla oblongata receives this input, it triggers a reflex that maintains homeostasis (Figure 20.18):
- When blood pressure rises too high, the baroreceptors fire at a higher rate and trigger parasympathetic stimulation of the heart. As a result, cardiac output falls. Sympathetic stimulation of the peripheral arterioles will also decrease, resulting in vasodilation. Combined, these activities cause blood pressure to fall.
- When blood pressure drops too low, the rate of baroreceptor firing decreases. This will trigger an increase in sympathetic stimulation of the heart, causing cardiac output to increase. It will also trigger sympathetic stimulation of the peripheral vessels, resulting in vasoconstriction. Combined, these activities cause blood pressure to rise.
Figure 20.18 Baroreceptor Reflexes for Maintaining Vascular Homeostasis Increased blood pressure results in increased rates of baroreceptor firing, whereas decreased blood pressure results in slower rates of fire, both initiating the homeostatic mechanism to restore blood pressure.
The baroreceptors in the venae cavae and right atrium monitor blood pressure as the blood returns to the heart from the systemic circulation. Normally, blood flow into the aorta is the same as blood flow back into the right atrium. If blood is returning to the right atrium more rapidly than it is being ejected from the left ventricle, the atrial receptors will stimulate the cardiovascular centers to increase sympathetic firing and increase cardiac output until homeostasis is achieved. The opposite is also true. This mechanism is referred to as the atrial reflex.
Chemoreceptor Reflexes
In addition to the baroreceptors are chemoreceptors that monitor levels of oxygen, carbon dioxide, and hydrogen ions (pH), and thereby contribute to vascular homeostasis. Chemoreceptors monitoring the blood are located in close proximity to the baroreceptors in the aortic and carotid sinuses. They signal the cardiovascular center as well as the respiratory centers in the medulla oblongata.
Since tissues consume oxygen and produce carbon dioxide and acids as waste products, when the body is more active, oxygen levels fall and carbon dioxide levels rise as cells undergo cellular respiration to meet the energy needs of activities. This causes more hydrogen ions to be produced, causing the blood pH to drop. When the body is resting, oxygen levels are higher, carbon dioxide levels are lower, more hydrogen is bound, and pH rises.
The chemoreceptors respond to increasing carbon dioxide and hydrogen ion levels (falling pH) by stimulating the cardioaccelerator and vasomotor centers, increasing cardiac output and constricting peripheral vessels. The cardioinhibitor centers are suppressed. With falling carbon dioxide and hydrogen ion levels (increasing pH), the cardioinhibitor centers are stimulated, and the cardioaccelerator and vasomotor centers are suppressed, decreasing cardiac output and causing peripheral vasodilation. In order to maintain adequate supplies of oxygen to the cells and remove waste products such as carbon dioxide, it is essential that the respiratory system respond to changing metabolic demands. In turn, the cardiovascular system will transport these gases to the lungs for exchange, again in accordance with metabolic demands. This interrelationship of cardiovascular and respiratory control cannot be overemphasized.
Other neural mechanisms can also have a significant impact on cardiovascular function. These include the limbic system that links physiological responses to psychological stimuli, as well as generalized sympathetic and parasympathetic stimulation.
[20.4.1] Endocrine Regulation
Endocrine control over the cardiovascular system involves the catecholamines, epinephrine and norepinephrine, as well as several hormones that interact with the kidneys in the regulation of blood volume.
Epinephrine and Norepinephrine
The catecholamines epinephrine and norepinephrine are released by the adrenal medulla, and enhance and extend the body’s sympathetic or “fight-or-flight” response (see Figure 20.17). They increase heart rate and force of contraction, while temporarily constricting blood vessels to organs not essential for flight-or-fight responses and redirecting blood flow to the liver, muscles, and heart.
Antidiuretic Hormone
Antidiuretic hormone (ADH), also known as vasopressin, is secreted by the cells in the hypothalamus and transported via the hypothalamic-hypophyseal tracts to the posterior pituitary where it is stored until released upon nervous stimulation. The primary trigger prompting the hypothalamus to release ADH is increasing osmolarity of tissue fluid, usually in response to significant loss of blood volume. ADH signals its target cells in the kidneys to reabsorb more water, thus preventing the loss of additional fluid in the urine. This will increase overall fluid levels and help restore blood volume and pressure. In addition, ADH constricts peripheral vessels.
Renin-Angiotensin-Aldosterone Mechanism
The renin-angiotensin-aldosterone mechanism has a major effect upon the cardiovascular system (Figure 20.19). Renin is an enzyme, although because of its importance in the renin-angiotensin-aldosterone pathway, some sources identify it as a hormone. Specialized cells in the kidneys found in the juxtaglomerular apparatus respond to decreased blood flow by secreting renin into the blood. Renin converts the plasma protein angiotensinogen, which is produced by the liver, into its active form—angiotensin I. Angiotensin I circulates in the blood and is then converted into angiotensin II in the lungs. This reaction is catalyzed by the enzyme angiotensin-converting enzyme (ACE).
Angiotensin II is a powerful vasoconstrictor, greatly increasing blood pressure. It also stimulates the release of ADH and aldosterone, a hormone produced by the adrenal cortex. Aldosterone increases the reabsorption of sodium into the blood by the kidneys. Since water follows sodium, this increases the reabsorption of water. This in turn increases blood volume, raising blood pressure. Angiotensin II also stimulates the thirst center in the hypothalamus, so an individual will likely consume more fluids, again increasing blood volume and pressure.
Figure 20.19 Hormones Involved in Renal Control of Blood Pressure In the renin-angiotensin-aldosterone mechanism, increasing angiotensin II will stimulate the production of antidiuretic hormone and aldosterone. In addition to renin, the kidneys produce erythropoietin, which stimulates the production of red blood cells, further increasing blood volume.
Erythropoietin
Erythropoietin (EPO) is released by the kidneys when blood flow and/or oxygen levels decrease. EPO stimulates the production of erythrocytes within the bone marrow. Erythrocytes are the major formed element of the blood and may contribute 40% or more to blood volume, a significant factor of viscosity, resistance, pressure, and flow. In addition, EPO is a vasoconstrictor. Overproduction of EPO or excessive intake of synthetic EPO, often to enhance athletic performance, will increase viscosity, resistance, and pressure, and decrease flow in addition to its contribution as a vasoconstrictor.
Atrial Natriuretic Hormone
Secreted by cells in the atria of the heart, atrial natriuretic hormone (ANH) (also known as atrial natriuretic peptide) is secreted when blood volume is high enough to cause extreme stretching of the cardiac cells. Cells in the ventricle produce a hormone with similar effects, called B-type natriuretic hormone. Natriuretic hormones are antagonists to angiotensin II. They promote loss of sodium and water from the kidneys, and suppress renin, aldosterone, and ADH production and release. All of these actions promote loss of fluid from the body, so blood volume and blood pressure drop.
[20.4.2] Autoregulation of Perfusion
As the name would suggest, autoregulation mechanisms require neither specialized nervous stimulation nor endocrine control. Rather, these are local, self-regulatory mechanisms that allow each region of tissue to adjust its blood flow—and thus its perfusion. These local mechanisms include chemical signals and myogenic controls.
Chemical Signals Involved in Autoregulation
Chemical signals work at the level of the precapillary sphincters to trigger either constriction or relaxation. As you know, opening a precapillary sphincter allows blood to flow into that particular capillary, whereas constricting a precapillary sphincter temporarily shuts off blood flow to that region. The factors involved in regulating the precapillary sphincters include the following:
- Opening of the sphincter is triggered in response to decreased oxygen concentrations; increased carbon dioxide concentrations; increasing levels of lactic acid or other byproducts of cellular metabolism; increasing concentrations of potassium ions or hydrogen ions (falling pH); inflammatory chemicals such as histamines; and increased body temperature. These conditions in turn stimulate the release of NO, a powerful vasodilator, from endothelial cells (see Figure 20.17).
- Contraction of the precapillary sphincter is triggered by the opposite levels of the regulators, which prompt the release of endothelins, powerful vasoconstricting peptides secreted by endothelial cells. Platelet secretions and certain prostaglandins may also trigger constriction.
Again, these factors alter tissue perfusion via their effects on the precapillary sphincter mechanism, which regulates blood flow to capillaries. Since the amount of blood is limited, not all capillaries can fill at once, so blood flow is allocated based upon the needs and metabolic state of the tissues as reflected in these parameters. Bear in mind, however, that dilation and constriction of the arterioles feeding the capillary beds is the primary control mechanism.
The Myogenic Response
The myogenic response is a reaction to the stretching of the smooth muscle in the walls of arterioles as changes in blood flow occur through the vessel. This may be viewed as a largely protective function against dramatic fluctuations in blood pressure and blood flow to maintain homeostasis. If perfusion of an organ is too low (ischemia), the tissue will experience low levels of oxygen (hypoxia). In contrast, excessive perfusion could damage the organ’s smaller and more fragile vessels. The myogenic response is a localized process that serves to stabilize blood flow in the capillary network that follows that arteriole.
When blood flow is low, the vessel’s smooth muscle will be only minimally stretched. In response, it relaxes, allowing the vessel to dilate and thereby increase the movement of blood into the tissue. When blood flow is too high, the smooth muscle will contract in response to the increased stretch, prompting vasoconstriction that reduces blood flow.
Figure 20.20 summarizes the effects of nervous, endocrine, and local controls on arterioles.
Figure 20.20 Summary of Mechanisms Regulating Arteriole Smooth Muscle and Veins.
[20.4.3] Effect of Exercise on Vascular Homeostasis
The heart is a muscle and, like any muscle, it responds dramatically to exercise. For a healthy young adult, cardiac output (heart rate × stroke volume) increases in the non-athlete from approximately 5.0 liters per minute to a maximum of about 20 liters per minute. Accompanying this will be an increase in blood pressure from about 120/80 mm Hg to 185/75 mm Hg. However, well-trained aerobic athletes can increase these values substantially. For these individuals, cardiac output soars from approximately 5.3 liters per minute resting to more than 30 liters per minute during maximal exercise. Along with this increase in cardiac output, blood pressure increases from 120/80 mm Hg at rest to 200/90 mm Hg at maximum values.
In addition to improved cardiac function, exercise increases the size and mass of the heart. The average weight of the heart for the non-athlete is about 300 g, whereas in an athlete it will increase to 500 g. This increase in size generally makes the heart stronger and more efficient at pumping blood, increasing both stroke volume and cardiac output.
Tissue perfusion also increases as the body transitions from a resting state to light exercise and eventually to heavy exercise (see Figure 20.20). These changes result in selective vasodilation in the skeletal muscles, heart, lungs, liver, and integument. Simultaneously, vasoconstriction occurs in the vessels leading to the kidneys and most of the digestive and reproductive organs. The flow of blood to the brain remains largely unchanged whether at rest or exercising, since the vessels in the brain largely do not respond to regulatory stimuli, in most cases, because they lack the appropriate receptors.
As vasodilation occurs in selected vessels, resistance drops and more blood rushes into the organs they supply. This blood eventually returns to the venous system. Venous return is further enhanced by both the skeletal muscle and respiratory pumps. As blood returns to the heart more quickly, preload rises and the Frank-Starling principle tells us that contraction of the cardiac muscle in the atria and ventricles will be more forceful. Eventually, even the best-trained athletes will fatigue and must undergo a period of rest following exercise. Cardiac output and distribution of blood then return to normal.
Regular exercise promotes cardiovascular health in a variety of ways. Because an athlete’s heart is larger than a nonathlete’s, stroke volume increases, so the athletic heart can deliver the same amount of blood as the nonathletic heart but with a lower heart rate. This increased efficiency allows the athlete to exercise for longer periods of time before muscles fatigue and places less stress on the heart. Exercise also lowers overall cholesterol levels by removing from the circulation a complex form of cholesterol, triglycerides, and proteins known as low-density lipoproteins (LDLs), which are widely associated with increased risk of cardiovascular disease. Although there is no way to remove deposits of plaque from the walls of arteries other than specialized surgery, exercise does promote the health of vessels by decreasing the rate of plaque formation and reducing blood pressure, so the heart does not have to generate as much force to overcome resistance.
Generally as little as 30 minutes of noncontinuous exercise over the course of each day has beneficial effects and has been shown to lower the rate of heart attack by nearly 50%. While it is always advisable to follow a healthy diet, stop smoking, and lose weight, studies have clearly shown that fit, overweight people may actually be healthier overall than sedentary slender people. Thus, the benefits of moderate exercise are undeniable.
Clinical Connections: Vascular Homeostasis
Any disorder that affects blood volume, vascular tone, or any other aspect of vascular functioning is likely to affect vascular homeostasis as well. That includes hypertension, hemorrhage, and shock.
Hypertension and Hypotension
Chronically elevated blood pressure is known clinically as hypertension. It is defined as chronic and persistent blood pressure measurements of 140/90 mm Hg or above. Pressures between 120/80 and 140/90 mm Hg are defined as prehypertension. Unfortunately, hypertension is typically a silent disorder; therefore, hypertensive patients may fail to recognize the seriousness of their condition and fail to follow their treatment plan. The result is often a heart attack or stroke. Hypertension may also lead to an aneurysm (ballooning of a blood vessel caused by a weakening of the wall), peripheral arterial disease (obstruction of vessels in peripheral regions of the body), chronic kidney disease, or heart failure.
Listen to this CDC podcast (http://openstaxcollege.org/l/CDCpodcast) to learn about hypertension, often described as a “silent killer.” What steps can you take to reduce your risk of a heart attack or stroke?
Hemorrhage
Minor blood loss is managed by hemostasis and repair. Hemorrhage is a loss of blood that cannot be controlled by hemostatic mechanisms. Initially, the body responds to hemorrhage by initiating mechanisms aimed at increasing blood pressure and maintaining blood flow. Ultimately, however, blood volume will need to be restored, either through physiological processes or through medical intervention.
In response to blood loss, stimuli from the baroreceptors trigger the cardiovascular centers to stimulate sympathetic responses to increase cardiac output and vasoconstriction. This typically prompts the heart rate to increase to about 180–200 contractions per minute, restoring cardiac output to normal levels. Vasoconstriction of the arterioles increases vascular resistance, whereas constriction of the veins increases venous return to the heart. Both of these steps will help increase blood pressure. Sympathetic stimulation also triggers the release of epinephrine and norepinephrine, which enhance both cardiac output and vasoconstriction. If blood loss were less than 20% of total blood volume, these responses together would usually return blood pressure to normal and redirect the remaining blood to the tissues.
Additional endocrine involvement is necessary, however, to restore the lost blood volume. The angiotensin-renin- aldosterone mechanism stimulates the thirst center in the hypothalamus, which increases fluid consumption to help restore the lost blood. More importantly, it increases renal reabsorption of sodium and water, reducing water loss in urine output. The kidneys also increase the production of EPO, stimulating the formation of erythrocytes that not only deliver oxygen to the tissues but also increase overall blood volume. Figure 20.21 summarizes the responses to loss of blood volume.
Figure 20.21 Homeostatic Responses to Loss of Blood Volume.
Circulatory Shock
The loss of too much blood may lead to circulatory shock, a life-threatening condition in which the circulatory system is unable to maintain blood flow to adequately supply sufficient oxygen and other nutrients to the tissues to maintain cellular metabolism. It should not be confused with emotional or psychological shock. Typically, the patient in circulatory shock will demonstrate an increased heart rate but decreased blood pressure, but there are cases in which blood pressure will remain normal. Urine output will fall dramatically, and the patient may appear confused or lose consciousness. Urine output less than 1 mL/kg body weight/hour is cause for concern. Unfortunately, shock is an example of a positive-feedback loop that, if uncorrected, may lead to the death of the patient.
There are several recognized forms of shock:
- Hypovolemic shock in adults is typically caused by hemorrhage, although in children it may be caused by fluid losses related to severe vomiting or diarrhea. Other causes for hypovolemic shock include extensive burns, exposure to some toxins, and excessive urine loss related to diabetes insipidus or ketoacidosis. Typically, patients present with a rapid, almost tachycardic heart rate; a weak pulse often described as “thread;” cool, clammy skin, particularly in the extremities, due to restricted peripheral blood flow; rapid, shallow breathing; hypothermia; thirst; and dry mouth. Treatments generally involve providing intravenous fluids to restore the patient to normal function and various drugs such as dopamine, epinephrine, and norepinephrine to raise blood pressure.
- Cardiogenic shock results from the inability of the heart to maintain cardiac output. Most often, it results from a myocardial infarction (heart attack), but it may also be caused by arrhythmias, valve disorders, cardiomyopathies, cardiac failure, or simply insufficient flow of blood through the cardiac vessels. Treatment involves repairing the damage to the heart or its vessels to resolve the underlying cause, rather than treating cardiogenic shock directly.
- Vascular shock occurs when arterioles lose their normal muscular tone and dilate dramatically. It may arise from a variety of causes, and treatments almost always involve fluid replacement and medications, called inotropic or pressor agents, which restore tone to the muscles of the vessels. In addition, eliminating or at least alleviating the underlying cause of the condition is required. This might include antibiotics and antihistamines, or select steroids, which may aid in the repair of nerve damage. A common cause is sepsis (or septicemia), also called “blood poisoning,” which is a widespread bacterial infection that results in an organismal-level inflammatory response known as septic shock. Neurogenic shock is a form of vascular shock that occurs with cranial or spinal injuries that damage the cardiovascular centers in the medulla oblongata or the nervous fibers originating from this region. Anaphylactic shock is a severe allergic response that causes the widespread release of histamines, triggering vasodilation throughout the body.
- Obstructive shock, as the name would suggest, occurs when a significant portion of the vascular system is blocked. It is not always recognized as a distinct condition and may be grouped with cardiogenic shock, including pulmonary embolism and cardiac tamponade. Treatments depend upon the underlying cause and, in addition to administering fluids intravenously, often include the administration of anticoagulants, removal of fluid from the pericardial cavity, or air from the thoracic cavity, and surgery as required. The most common cause is a pulmonary embolism, a clot that lodges in the pulmonary vessels and interrupts blood flow. Other causes include stenosis of the aortic valve; cardiac tamponade, in which excess fluid in the pericardial cavity interferes with the ability of the heart to fully relax and fill with blood (resulting in decreased preload); and a pneumothorax, in which an excessive amount of air is present in the thoracic cavity, outside of the lungs, which interferes with venous return, pulmonary function, and delivery of oxygen to the tissues.
[20.5] Circulatory Pathways
Learning Objectives
By the end of this section, you will be able to:
- Identify the vessels through which blood travels within the pulmonary circuit, beginning from the right ventricle of the heart and ending at the left atrium
- Create a flow chart showing the major systemic arteries through which blood travels from the aorta and its major branches, to the most significant arteries feeding into the right and left upper and lower limbs
- Create a flow chart showing the major systemic veins through which blood travels from the feet to the right atrium of the heart
Virtually every cell, tissue, organ, and system in the body is impacted by the circulatory system. This includes the generalized and more specialized functions of transport of materials, capillary exchange, maintaining health by transporting white blood cells and various immunoglobulins (antibodies), hemostasis, regulation of body temperature, and helping to maintain acid-base balance. In addition to these shared functions, many systems enjoy a unique relationship with the circulatory system. Figure 20.22 summarizes these relationships.
Figure 20.22 Interaction of the Circulatory System with Other Body Systems
As you learn about the vessels of the systemic and pulmonary circuits, notice that many arteries and veins share the same names, parallel one another throughout the body, and are very similar on the right and left sides of the body. These pairs of vessels will be traced through only one side of the body. Where differences occur in branching patterns or when vessels are singular, this will be indicated. For example, you will find a pair of femoral arteries and a pair of femoral veins, with one vessel on each side of the body. In contrast, some vessels closer to the midline of the body, such as the aorta, are unique. Moreover, some superficial veins, such as the great saphenous vein in the femoral region, have no arterial counterpart. Another phenomenon that can make the study of vessels challenging is that names of vessels can change with location. Like a street that changes name as it passes through an intersection, an artery or vein can change names as it passes an anatomical landmark. For example, the left subclavian artery becomes the axillary artery as it passes through the body wall and into the axillary region, and then becomes the brachial artery as it flows from the axillary region into the upper arm (or brachium). You will also find examples of anastomoses where two blood vessels that previously branched reconnect. Anastomoses are especially common in veins, where they help maintain blood flow even when one vessel is blocked or narrowed, although there are some important ones in the arteries supplying the brain and heart.
As you read about circular pathways, notice that there is an occasional, very large artery referred to as a trunk, a term indicating that the vessel gives rise to several smaller arteries. For example, the celiac trunk gives rise to the left gastric, common hepatic, and splenic arteries.
Visit this site (http://openstaxcollege.org/l/arts1) for a brief summary of the arteries.
As you study this section, imagine you are on a “Voyage of Discovery” similar to Lewis and Clark’s expedition in 1804–1806, which followed rivers and streams through unfamiliar territory, seeking a water route from the Atlantic to the Pacific Ocean. You might envision being inside a miniature boat, exploring the various branches of the circulatory system. This simple approach has proven effective for many students in mastering these major circulatory patterns. Another approach that works well for many students is to create simple line drawings similar to the ones provided, labeling each of the major vessels. It is beyond the scope of this text to name every vessel in the body. However, we will attempt to discuss the major pathways for blood and acquaint you with the major named arteries and veins in the body. Also, please keep in mind that individual variations in circulation patterns are not uncommon.
[20.5.1] Pulmonary Circulation
Recall that blood returning from the systemic circuit enters the right atrium (Figure 20.23) via the superior and inferior venae cavae and the coronary sinus, which drains the blood supply of the heart muscle. These vessels will be described more fully later in this section. This blood is relatively low in oxygen and relatively high in carbon dioxide, since much of the oxygen has been extracted for use by the tissues and the waste gas carbon dioxide was picked up to be transported to the lungs for elimination. From the right atrium, blood moves into the right ventricle, which pumps it to the lungs for gas exchange. This system of vessels is referred to as the pulmonary circuit.
The single vessel exiting the right ventricle is the pulmonary trunk. At the base of the pulmonary trunk is the pulmonary semilunar valve, which prevents backflow of blood into the right ventricle during ventricular diastole. As the pulmonary trunk reaches the superior surface of the heart, it curves posteriorly and rapidly bifurcates (divides) into two branches, a left and a right pulmonary artery. To prevent confusion between these vessels, it is important to refer to the vessel exiting the heart as the pulmonary trunk, rather than also calling it a pulmonary artery. The pulmonary arteries in turn branch many times within the lung, forming a series of smaller arteries and arterioles that eventually lead to the pulmonary capillaries. The pulmonary capillaries surround lung structures known as alveoli that are the sites of oxygen and carbon dioxide exchange.
Once gas exchange is completed, oxygenated blood flows from the pulmonary capillaries into a series of pulmonary venules that eventually lead to a series of larger pulmonary veins. Four pulmonary veins, two on the left and two on the right, return blood to the left atrium. At this point, the pulmonary circuit is complete. Table 20.4 defines the major arteries and veins of the pulmonary circuit discussed in the text.
Figure 20.23 Pulmonary Circuit Blood exiting from the right ventricle flows into the pulmonary trunk, which bifurcates into the two pulmonary arteries. These vessels branch to supply blood to the pulmonary capillaries, where gas exchange occurs within the lung alveoli. Blood returns via the pulmonary veins to the left atrium.
Pulmonary Arteries and Veins
| Vessel | Description |
| Pulmonary trunk | Single large vessel exiting the right ventricle that divides to form the right and left pulmonary arteries |
| Pulmonary arteries | Left and right vessels that form from the pulmonary trunk and lead to smaller arterioles and eventually to the pulmonary capillaries |
| Pulmonary veins | Two sets of paired vessels—one pair on each side—that are formed from the small venules, leading away from the pulmonary capillaries to flow into the left atrium |
Table 20.4
Overview of Systemic Arteries
Blood relatively high in oxygen concentration is returned from the pulmonary circuit to the left atrium via the four pulmonary veins. From the left atrium, blood moves into the left ventricle, which pumps blood into the aorta. The aorta and its branches—the systemic arteries—send blood to virtually every organ of the body (Figure 20.24).
Figure 20.24 Systemic Arteries The major systemic arteries shown here deliver oxygenated blood throughout the body.
[20.5.2] The Aorta
The aorta is the largest artery in the body (Figure 20.25). It arises from the left ventricle and eventually descends to the abdominal region, where it terminally bifurcates at the level of the fourth lumbar vertebra into the two common iliac arteries. The aorta consists of the ascending aorta, the aortic arch, and the descending aorta, which passes through the thoracic diaphragm, a landmark that divides into the superior thoracic and inferior abdominal components. Arteries originating from the aorta ultimately distribute blood to virtually all tissues of the body. At the base of the aorta is the aortic semilunar valve that prevents backflow of blood into the left ventricle while the heart is relaxing. After exiting the heart, the ascending aorta moves in a superior direction for approximately 5 cm and ends at the sternal angle. Following this ascent, it reverses direction, forming a graceful arc to the left, called the aortic arch. The aortic arch descends toward the inferior portions of the body and ends at the level of the intervertebral disk between the fourth and fifth thoracic vertebrae. Beyond this point, the descending aorta continues close to the bodies of the vertebrae and passes through an opening in the thoracic diaphragm known as the aortic hiatus. Superior to the thoracic diaphragm, the aorta is called the thoracic aorta, and inferior to the thoracic diaphragm, it is called the abdominal aorta. The abdominal aorta terminates when it bifurcates into the two common iliac arteries at the level of the fourth lumbar vertebra.
See Figure 20.25 for an illustration of the ascending aorta, the aortic arch, and the initial segment of the descending aorta plus major branches; Table 20.5 summarizes the structures of the aorta.
Figure 20.25 Aorta The aorta has distinct regions, including the ascending aorta, aortic arch, and the descending aorta, which includes the thoracic and abdominal regions.
Components of the Aorta
| Vessel | Description |
| Aorta | Largest artery in the body, originating from the left ventricle and descending to the abdominal region, where it bifurcates into the common iliac arteries at the level of the fourth lumbar vertebra; arteries originating from the aorta distribute blood to virtually all tissues of the body |
| Ascending aorta | Initial portion of the aorta, rising superiorly from the left ventricle for a distance of approximately 5 cm |
| Aortic arch | Graceful arc to the left that connects the ascending aorta to the descending aorta; ends at the intervertebral disk between the fourth and fifth thoracic vertebrae |
| Descending aorta | Portion of the aorta that continues inferiorly past the end of the aortic arch; subdivided into the thoracic aorta and the abdominal aorta |
| Thoracic aorta | Portion of the descending aorta superior to the aortic hiatus |
| Abdominal aorta | Portion of the aorta inferior to the aortic hiatus and superior to the common iliac arteries |
Table 20.5
Coronary Circulation
The first vessels that branch from the ascending aorta are the paired coronary arteries (see Figure 20.25), which arise from two of the three sinuses in the ascending aorta just superior to the aortic semilunar valve. These sinuses contain the aortic baroreceptors and chemoreceptors critical to maintain cardiac function. The left coronary artery arises from the left posterior aortic sinus. The right coronary artery arises from the anterior aortic sinus. Normally, the right posterior aortic sinus does not give rise to a vessel.
The coronary arteries encircle the heart, forming a ring-like structure that divides into the next level of branches that supplies blood to the heart tissues. For more detail on the coronary circulation see Chapter 19.
Aortic Arch Branches
There are three major branches of the aortic arch: the brachiocephalic artery, the left common carotid artery, and the left subclavian (literally “under the clavicle”) artery. As you would expect based upon proximity to the heart, each of these vessels is classified as an elastic artery.
The brachiocephalic artery is located only on the right side of the body; there is no corresponding artery on the left. The brachiocephalic artery branches into the right subclavian artery and the right common carotid artery. The left subclavian and left common carotid arteries arise independently from the aortic arch but otherwise follow a similar pattern and distribution to the corresponding arteries on the right side (see Figure 20.23).
Each subclavian artery supplies blood to the arms, chest, shoulders, back, and central nervous system. It then gives rise to three major branches: the internal thoracic artery, the vertebral artery, and the thyrocervical artery. The vertebral artery passes through the vertebral foramen in the cervical vertebrae and then through the foramen magnum into the cranial cavity to supply blood to the brain and spinal cord. The paired vertebral arteries join together to form the large basilar artery at the base of the medulla oblongata.
The common carotid artery divides into internal and external carotid arteries. The right common carotid artery arises from the brachiocephalic artery and the left common carotid artery arises directly from the aortic arch. The external carotid artery supplies blood to numerous structures within the face, lower jaw, neck, esophagus, and larynx. The internal carotid artery initially forms an expansion known as the carotid sinus, containing the carotid baroreceptors and chemoreceptors. Like their counterparts in the aortic sinuses, the information provided by these receptors is critical to maintaining cardiovascular homeostasis (see Figure 20.23).
The internal carotid arteries along with the vertebral arteries are the two primary suppliers of blood to the human brain. Given the central role and vital importance of the brain to life, it is critical that blood supply to this organ remains uninterrupted. Recall that blood flow to the brain is remarkably constant, with approximately 20% of blood flow directed to this organ at any given time. When blood flow is interrupted, even for just a few seconds, a transient ischemic attack (TIA), or mini-stroke, may occur, resulting in loss of consciousness or temporary loss of neurological function. In some cases, the damage may be permanent. Loss of blood flow for longer periods, typically between 3 and 4 minutes, will likely produce irreversible brain damage or a stroke, also called a cerebrovascular accident (CVA). The locations of the arteries in the brain not only provide blood flow to the brain tissue but also prevent interruption in the flow of blood. Both the carotid and vertebral arteries branch once they enter the cranial cavity, and some of these branches form a structure known as the cerebral arterial circle (or circle of Willis), an anastomosis that is remarkably like a traffic circle that sends off branches (in this case, arterial branches to the brain). As a rule, branches to the anterior portion of the cerebrum are normally fed by the internal carotid arteries; the remainder of the brain receives blood flow from branches associated with the vertebral arteries. The circle of Willis is an important means of establishing alternative routes of blood flow to the brain in the cranial cavity. Establishing such alternative routes offers redundancy in blood flow and potentially limits tissue damage in the event that one of the vessels supplying blood to the brain becomes occluded (see Figure 20.27).
Table 20.6 summarises the aortic arch branches, including the major branches supplying the brain.
Figure 20.26 Arteries Supplying the Head and Neck The common carotid artery gives rise to the external and internal carotid arteries. The external carotid artery remains superficial and gives rise to many arteries of the head. The internal carotid artery first forms the carotid sinus and then reaches the brain via the carotid canal and carotid foramen, emerging into the cranium via the foramen lacerum. The vertebral artery branches from the subclavian artery and passes through the transverse foramen in the cervical vertebrae, entering the base of the skull at the vertebral foramen. The subclavian artery continues toward the arm as the axillary artery.
Figure 20.27 Arteries Serving the Brain This inferior view shows the network of arteries serving the brain. The structure is referred to as the arterial circle or circle of Willis.
Aortic Arch Branches and Brain Circulation
| Vessel | Description |
| Brachiocephalic artery | Single vessel located on the right side of the body; the first vessel branching from the aortic arch; gives rise to the right subclavian artery and the right common carotid artery; supplies blood to the head, neck, upper limb, and wall of the thoracic region |
| Subclavian artery | The right subclavian artery arises from the brachiocephalic artery while the left subclavian artery arises from the aortic arch; gives rise to the internal thoracic, vertebral, and thyrocervical arteries; supplies blood to the arms, chest, shoulders, back, and central nervous system |
| Internal thoracic artery | Also called the mammary artery; arises from the subclavian artery; supplies blood to the thymus, pericardium of the heart, and anterior chest wall |
| Vertebral artery | Arises from the subclavian artery and passes through the vertebral foramen through the foramen magnum to the brain; joins with the internal carotid artery to form the arterial circle; supplies blood to the brain and spinal cord |
| Thyrocervical artery | Arises from the subclavian artery; supplies blood to the thyroid, the cervical region, the upper back, and shoulder |
| Common carotid artery | The right common carotid artery arises from the brachiocephalic artery and the left common carotid artery arises from the aortic arch; each gives rise to the external and internal carotid arteries; supplies the respective sides of the head and neck |
| External carotid artery | Arises from the common carotid artery; supplies blood to numerous structures within the face, lower jaw, neck, esophagus, and larynx |
| Internal carotid artery | Arises from the common carotid artery and begins with the carotid sinus; goes through the carotid canal of the temporal bone to the base of the brain; combines with the branches of the vertebral artery, forming the arterial circle; supplies blood to the brain |
| Arterial circle or circle of Willis | An anastomosis located at the base of the brain that ensures continual blood supply; formed from the branches of the internal carotid and vertebral arteries; supplies blood to the brain |
Table 20.6
Thoracic Aorta and Major Branches
The thoracic aorta begins at the level of vertebra T5 and continues through to the thoracic diaphragm at the level of T12, initially traveling within the mediastinum to the left of the vertebral column. As it passes through the thoracic region, the thoracic aorta gives rise to several branches, which are collectively referred to as visceral branches and parietal branches (Figure 20.28). Those branches that supply blood primarily to visceral organs are known as the visceral branches and include the bronchial arteries, pericardial arteries, esophageal arteries, and the mediastinal arteries, each named after the tissues it supplies. Each bronchial artery (typically two on the left and one on the right) supplies systemic blood to the lungs and visceral pleura, in addition to the blood pumped to the lungs for oxygenation via the pulmonary circuit. This may sound incongruous—that is, the mixing of systemic arterial blood high in oxygen with the pulmonary arterial blood lower in oxygen—but the systemic vessels also deliver nutrients to the lung tissue just as they do elsewhere in the body. The remaining thoracic aorta branches are collectively referred to as parietal branches, and include a number of branches which provide blood to the thoracic wall and superior surface of the thoracic diaphragm. Table 20.7 lists the arteries of the thoracic region.
Figure 20.28 Arteries of the Thoracic and Abdominal Regions The thoracic aorta gives rise to the arteries of the visceral and parietal branches.
Arteries of the Thoracic Region
| Vessel | Description |
| Visceral branches | A group of arterial branches of the thoracic aorta; supplies blood to the viscera (i.e., organs) of the thorax |
| Bronchial artery | Systemic branch from the aorta that provides oxygenated blood to the lungs; this blood supply is in addition to the pulmonary circuit that brings blood for oxygenation |
| Pericardial artery | Branch of the thoracic aorta; supplies blood to the pericardium |
| Esophageal artery | Branch of the thoracic aorta; supplies blood to the esophagus |
| Mediastinal artery | Branch of the thoracic aorta; supplies blood to the mediastinum |
| Parietal branches | Also called somatic branches, a group of arterial branches of the thoracic aorta; include those that supply blood to the thoracic wall, vertebral column, and the superior surface of the diaphragm |
| Intercostal artery | Branch of the thoracic aorta; supplies blood to the muscles of the thoracic cavity and vertebral column |
| Superior phrenic artery | Branch of the thoracic aorta; supplied blood to the superior surface of the thoracic diaphragm |
Table 20.7
Abdominal Aorta and Major Branches
After crossing through the thoracic diaphragm at the aortic hiatus, the thoracic aorta is called the abdominal aorta (see Figure 20.28). This vessel remains to the left of the vertebral column and is embedded in adipose tissue behind the peritoneal cavity. It formally ends at approximately the level of vertebra L4, where it bifurcates to form the common iliac arteries. Before this division, the abdominal aorta gives rise to several important branches. A single coeliac trunk (artery) emerges and divides into the left gastric artery to supply blood to the stomach and esophagus, the splenic artery to supply blood to the spleen, and the common hepatic artery, which in turn gives rise to the hepatic artery proper to supply blood to the liver, the right gastric artery to supply blood to the stomach, the cystic artery to supply blood to the gall bladder, and several branches, one to supply blood to the duodenum and another to supply blood to the pancreas. Two additional single vessels arise from the abdominal aorta. These are the superior and inferior mesenteric arteries. The superior mesenteric artery arises approximately 2.5 cm after the celiac trunk and branches into several major vessels that supply blood to the small intestine (duodenum, jejunum, and ileum), the pancreas, and a majority of the large intestine. The inferior mesenteric artery supplies blood to the distal segment of the large intestine, including the rectum. It arises approximately 5 cm superior to the common iliac arteries.
In addition to these single branches, the abdominal aorta gives rise to several significant paired arteries along the way. These include the inferior phrenic arteries, the adrenal arteries, the renal arteries, the gonadal arteries, and the lumbar arteries. Each renal artery branches approximately 2.5 cm inferior to the superior mesenteric arteries and supplies a kidney. The right renal artery is longer than the left since the aorta lies to the left of the vertebral column and the vessel must travel a greater distance to reach its target. Renal arteries branch repeatedly to supply blood to the kidneys. Each gonadal artery supplies blood to the gonads, or reproductive organs, and is also described as either an ovarian artery or a testicular artery (internal spermatic), depending upon the sex of the individual. An ovarian artery supplies blood to an ovary, uterine (Fallopian) tube, and the uterus, and is located within the suspensory ligament of the uterus. It is considerably shorter than a testicular artery, which ultimately travels outside the body cavity to the testes, forming one component of the spermatic cord. The gonadal arteries arise inferior to the renal arteries and are generally retroperitoneal. The ovarian artery continues to the uterus where it forms an anastomosis with the uterine artery that supplies blood to the uterus. Both the uterine arteries and vaginal arteries, which distribute blood to the vagina, are branches of the internal iliac artery.
The aorta divides at approximately the level of vertebra L4 into a left and a right common iliac artery. The common iliac arteries provide blood to the pelvic region and ultimately to the lower limbs. They split into external and internal iliac arteries approximately at the level of the lumbar-sacral articulation. Each internal iliac artery sends branches to the urinary bladder, the walls of the pelvis, the external genitalia, and the medial portion of the femoral region. In females, they also provide blood to the uterus and vagina. The much larger external iliac artery supplies blood to each of the lower limbs. Figure 20.29 shows the distribution of the major branches of the aorta into the thoracic and abdominal regions. Figure 20.30 shows the distribution of the major branches of the common iliac arteries. Table 20.8 summarizes the major branches of the abdominal aorta.
Figure 20.29 Major Branches of the Aorta The flow chart summarizes the distribution of the major branches of the aorta into the thoracic and abdominal regions.
Figure 20.30 Major Branches of the Iliac Arteries The flow chart summarizes the distribution of the major branches of the common iliac arteries into the pelvis and lower limbs. The left side follows a similar pattern to the right.
Vessels of the Abdominal Aorta
| Vessel | Description |
| Celiac trunk | Also called the celiac artery; a major branch of the abdominal aorta; gives rise to the left gastric artery, the splenic artery, and the common hepatic artery that forms the hepatic artery to the liver, the right gastric artery to the stomach, and the cystic artery to the gall bladder |
| Left gastric artery | Branch of the celiac trunk; supplies blood to the stomach |
| Splenic artery | Branch of the celiac trunk; supplies blood to the spleen |
| Common hepatic artery | Branch of the celiac trunk that forms the hepatic artery, the right gastric artery, and the cystic artery |
| Hepatic artery proper | Branch of the common hepatic artery; supplies systemic blood to the liver |
| Right gastric artery | Branch of the common hepatic artery; supplies blood to the stomach |
| Cystic artery | Branch of the common hepatic artery; supplies blood to the gall bladder |
| Superior mesenteric artery | Branch of the abdominal aorta; supplies blood to the small intestine (duodenum, jejunum, and ileum), the pancreas, and a majority of the large intestine |
| Inferior mesenteric artery | Branch of the abdominal aorta; supplies blood to the distal segment of the large intestine and rectum |
| Inferior phrenic arteries | Branches of the abdominal aorta; supply blood to the inferior surface of the diaphragm |
| Adrenal artery | Branch of the abdominal aorta; supplies blood to the adrenal (suprarenal) glands |
| Renal artery | Branch of the abdominal aorta; supplies each kidney |
| Gonadal artery | Branch of the abdominal aorta; supplies blood to the gonads or reproductive organs; also described as ovarian arteries or testicular arteries, depending upon the sex of the individual |
| Ovarian artery | Branch of the abdominal aorta; supplies blood to ovary, uterine (Fallopian) tube, and uterus |
| Testicular artery | Branch of the abdominal aorta; ultimately travels outside the body cavity to the testes and forms one component of the spermatic cord |
| Lumbar arteries | Branches of the abdominal aorta; supply blood to the lumbar region, the abdominal wall, and spinal cord |
| Common iliac artery | Branch of the aorta that leads to the internal and external iliac arteries |
| Median sacral artery | Continuation of the aorta into the sacrum |
| Internal iliac artery | Branch from the common iliac arteries; supplies blood to the urinary bladder, walls of the pelvis, external genitalia, and the medial portion of the femoral region; in females, also provides blood to the uterus and vagina |
| External iliac artery | Branch of the common iliac artery that leaves the body cavity and becomes a femoral artery; supplies blood to the lower limbs |
Table 20.8
Arteries Serving the Upper Limbs
As the subclavian artery exits the thorax into the axillary region, it is renamed the axillary artery. Although it does branch and supply blood to the region near the head of the humerus (via the humeral circumflex arteries), the majority of the vessel continues into the upper arm, or brachium, and becomes the brachial artery (Figure 20.31). The brachial artery supplies blood to much of the brachial region and divides at the elbow into several smaller branches, including the deep brachial arteries, which provide blood to the posterior surface of the arm, and the ulnar collateral arteries, which supply blood to the region of the elbow. As the brachial artery approaches the coronoid fossa, it bifurcates into the radial and ulnar arteries, which continue into the forearm, or antebrachium. The radial artery and ulnar artery parallel their namesake bones, giving off smaller branches until they reach the wrist, or carpal region. At this level, they fuse to form the superficial and deep palmar arches that supply blood to the hand, as well as the digital arteries that supply blood to the digits. Figure 20.32 shows the distribution of systemic arteries from the heart into the upper limb. Table 20.9 summarizes the arteries serving the upper limbs.
Figure 20.31 Major Arteries Serving the Thorax and Upper Limb The arteries that supply blood to the arms and hands are extensions of the subclavian arteries.
Figure 20.32 Major Arteries of the Upper Limb The flow chart summarizes the distribution of the major arteries from the heart into the upper limb.
Arteries Serving the Upper Limbs
| Vessel | Description |
| Axillary artery | Continuation of the subclavian artery as it penetrates the body wall and enters the axillary region; supplies blood to the region near the head of the humerus (humeral circumflex arteries); the majority of the vessel continues into the brachium and becomes the brachial artery |
| Brachial artery | Continuation of the axillary artery in the brachium; supplies blood to much of the brachial region; gives off several smaller branches that provide blood to the posterior surface of the arm in the region of the elbow; bifurcates into the radial and ulnar arteries at the coronoid fossa |
| Radial artery | Formed at the bifurcation of the brachial artery; parallels the radius; gives off smaller branches until it reaches the carpal region where it fuses with the ulnar artery to form the superficial and deep palmar arches; supplies blood to the lower arm and carpal region |
| Ulnar artery | Formed at the bifurcation of the brachial artery; parallels the ulna; gives off smaller branches until it reaches the carpal region where it fuses with the radial artery to form the superficial and deep palmar arches; supplies blood to the lower arm and carpal region |
| Palmar arches (superficial and deep) | Formed from anastomosis of the radial and ulnar arteries; supply blood to the hand and digital arteries |
| Digital arteries | Formed from the superficial and deep palmar arches; supply blood to the digits |
Table 20.9
Arteries Serving the Lower Limbs
The external iliac artery exits the body cavity and enters the femoral region of the lower leg (Figure 20.33). As it passes through the body wall, it is renamed the femoral artery. It gives off several smaller branches as well as the lateral deep femoral artery that supplies blood to the muscles of the posterior thigh. As the femoral artery passes posterior to the knee near the popliteal fossa, it is called the popliteal artery. The popliteal artery branches into the anterior and posterior tibial arteries.
The anterior tibial artery is located between the tibia and fibula, and supplies blood to the muscles and integument of the anterior tibial region. Upon reaching the tarsal region, it becomes the dorsalis pedis artery, which branches repeatedly and provides blood to the tarsal and dorsal regions of the foot. The posterior tibial artery provides blood to the muscles and integument on the posterior surface of the tibial region. Figure 20.34 shows the distribution of the major systemic arteries in the lower limb. Table 20.10 summarizes the major systemic arteries discussed in the text.
Figure 20.33 Major Arteries Serving the Lower Limb Major arteries serving the lower limb are shown in anterior and posterior views.
Figure 20.34 Systemic Arteries of the Lower Limb The flow chart summarizes the distribution of the systemic arteries from the external iliac artery into the lower limb.
Arteries Serving the Lower Limbs
| Vessel | Description |
| Femoral artery | Continuation of the external iliac artery after it passes through the body cavity; divides into several smaller branches, the lateral deep femoral artery, and the genicular artery; becomes the popliteal artery as it passes posterior to the knee |
| Deep femoral artery | Branch of the femoral artery; gives rise to the lateral circumflex arteries |
| Popliteal artery | Continuation of the femoral artery posterior to the knee; branches into the anterior and posterior tibial arteries |
| Anterior tibial artery | Branches from the popliteal artery; supplies blood to the anterior tibial region; becomes the dorsalis pedis artery |
| Dorsalis pedis artery | Forms from the anterior tibial artery; branches repeatedly to supply blood to the tarsal and dorsal regions of the foot |
| Posterior tibial artery | Branches from the popliteal artery and gives rise to the fibular or peroneal artery; supplies blood to the posterior tibial region |
Table 20.10
Overview of Systemic Veins
Systemic veins return blood to the right atrium. Since the blood has already passed through the systemic capillaries, it will be relatively low in oxygen concentration. In many cases, there will be veins draining organs and regions of the body with the same name as the arteries that supplied these regions and the two often parallel one another. This is often described as a “complementary” pattern. However, there is a great deal more variability in the venous circulation than normally occurs in the arteries. For the sake of brevity and clarity, this text will discuss only the most commonly encountered patterns. However, keep this variation in mind when you move from the classroom to clinical practice.
In both the neck and limb regions, there are often both superficial and deeper levels of veins. The deeper veins generally correspond to the complementary arteries. The superficial veins do not normally have direct arterial counterparts, but in addition to returning blood, they also make contributions to the maintenance of body temperature. When the ambient temperature is warm, more blood is diverted to the superficial veins where heat can be more easily dissipated to the environment. In colder weather, there is more constriction of the superficial veins and blood is diverted deeper where the body can retain more of the heat.
The “Voyage of Discovery” analogy and stick drawings mentioned earlier remain valid techniques for the study of systemic veins, but veins present a more difficult challenge because there are numerous anastomoses and multiple branches. It is like following a river with many tributaries and channels, several of which interconnect. Tracing blood flow through arteries follows the current in the direction of blood flow, so that we move from the heart through the large arteries and into the smaller arteries to the capillaries. From the capillaries, we move into the smallest veins and follow the direction of blood flow into larger veins and back to the heart. Figure 20.35 outlines the path of the major systemic veins.
Visit this site (http://openstaxcollege.org/l/veinsum) for a brief online summary of the veins.
Figure 20.35 Major Systemic Veins of the Body The major systemic veins of the body are shown here in an anterior view.
The right atrium receives all of the systemic venous return. Most of the blood flows into either the superior vena cava or inferior vena cava. If you draw an imaginary line at the level of the thoracic diaphragm, systemic venous circulation from above that line will generally flow into the superior vena cava; this includes blood from the head, neck, chest, shoulders, and upper limbs. The exception to this is that most venous blood flow from the coronary veins flows directly into the coronary sinus and from there directly into the right atrium. Beneath the thoracic diaphragm, systemic venous flow enters the inferior vena cava, that is, blood from the abdominal and pelvic regions and the lower limbs.
The Superior Vena Cava
The superior vena cava drains most of the body superior to the thoracic diaphragm (Figure 20.36). On both the left and right sides, the subclavian vein forms when the axillary vein passes through the body wall from the axillary region. It fuses with the external and internal jugular veins from the head and neck to form the brachiocephalic vein. Each vertebral vein also flows into the brachiocephalic vein close to this fusion. These veins arise from the base of the brain and the cervical region of the spinal cord, and flow largely through the intervertebral foramina in the cervical vertebrae. They are the counterparts of the vertebral arteries.
The remainder of the blood supply from the thorax drains into the azygos vein. Blood from the right and left sides of the thoracic wall flows through a number of tributaries to drain into the azygos vein and the smaller hemiazygos vein (hemi- = “half”), respectively. The hemiazygos vein does not drain directly into the superior vena cava but enters the brachiocephalic vein via the superior intercostal vein.
The azygos vein passes through the thoracic diaphragm from the thoracic cavity on the right side of the vertebral column and begins in the lumbar region of the abdominal cavity. It flows into the superior vena cava at approximately the level of T2, making a significant contribution to the flow of blood.
Table 20.11 summarises the veins of the thoracic region that flow into the superior vena cava.
Figure 20.36 Veins of the Thoracic and Abdominal Regions Veins of the thoracic and abdominal regions drain blood from the area above the diaphragm, returning it to the right atrium via the superior vena cava.
Veins of the Thoracic Region
| Vessel | Description |
| Superior vena cava | Large systemic vein; drains blood from most areas superior to the diaphragm; empties into the right atrium |
| Subclavian vein | Located deep in the thoracic cavity; formed by the axillary vein as it enters the thoracic cavity from the axillary region; drains the axillary and smaller local veins near the scapular region and leads to the brachiocephalic vein |
| Brachiocephalic veins | Pair of veins that form from a fusion of the external and internal jugular veins and the subclavian vein; subclavian, external and internal jugulars, vertebral, and internal thoracic veins flow into it; drain the upper thoracic region and lead to the superior vena cava |
| Vertebral vein | Arises from the base of the brain and the cervical region of the spinal cord; passes through the intervertebral foramina in the cervical vertebrae; drains smaller veins from the cranium, spinal cord, and vertebrae, and leads to the brachiocephalic vein; counterpart of the vertebral artery |
| Azygos vein | Originates in the lumbar region and passes through the thoracic diaphragm into the thoracic cavity on the right side of the vertebral column; drains blood from the intercostal veins, esophageal veins, bronchial veins, and other veins draining the mediastinal region, and leads to the superior vena cava |
| Hemiazygos vein | Smaller vein complementary to the azygos vein; drains the esophageal veins from the esophagus and the left intercostal veins, and leads to the brachiocephalic vein via the superior intercostal vein |
Table 20.11
Veins of the Head and Neck
Blood from the brain and the superficial facial vein flow into each internal jugular vein (Figure 20.37). Blood from the more superficial portions of the head, scalp, and cranial regions, flow into each external jugular vein. Although the external and internal jugular veins are separate vessels, there are anastomoses between them close to the thoracic region. Blood from the external jugular vein empties into the subclavian vein. Table 20.12 summarises the major veins of the head and neck.
Major Veins of the Head and Neck
| Vessel | Description |
| Internal jugular vein | Parallel to the common carotid artery, which is more or less its counterpart, and passes through the jugular foramen and canal; primarily drains blood from the brain, receives the superficial facial vein, and empties into the subclavian vein |
| External jugular vein | Drains blood from the more superficial portions of the head, scalp, and cranial regions, and leads to the subclavian vein |
Table 20.12
Venous Drainage of the Brain
Circulation to the brain is both critical and complex (see Figure 20.37). Many smaller veins of the brain stem and the superficial veins of the cerebrum lead to larger vessels referred to as intracranial sinuses. These include the superior and inferior sagittal sinuses, straight sinus, cavernous sinuses, left and right transverse sinuses, the petrosal sinuses, and the occipital sinuses. Ultimately, sinuses will lead back to either the inferior jugular vein or vertebral vein.
Most of the veins on the superior surface of the cerebrum flow into the largest of the sinuses, the superior sagittal sinus. It is located midsagittally between the meningeal and periosteal layers of the dura mater within the falx cerebri and, at first glance in images or models, can be mistaken for the subarachnoid space. Most reabsorption of cerebrospinal fluid occurs via the chorionic villi (arachnoid granulations) into the superior sagittal sinus. Blood from most of the smaller vessels originating from the inferior cerebral veins flows into the great cerebral vein and into the straight sinus. The sagittal sinus and straight sinuses all flow into the left and right transverse sinuses near the lambdoid suture. The transverse sinuses in turn flow into the sigmoid sinuses that pass through the jugular foramen and into the internal jugular vein. The internal jugular vein flows parallel to the common carotid artery and is more or less its counterpart. It empties into the brachiocephalic vein. The veins draining the cervical vertebrae and the posterior surface of the skull, including some blood from the occipital sinus, flow into the vertebral veins. These parallel the vertebral arteries and travel through the transverse foramina of the cervical vertebrae. The vertebral veins also flow into the brachiocephalic veins. Table 20.13 summarizes the major veins of the brain.
Figure 20.37 Veins of the Head and Neck This left lateral view shows the veins of the head and neck, including the intercranial sinuses.
Major Veins of the Brain
| Vessel | Description |
| Superior sagittal sinus | Enlarged vein located midsagittally between the meningeal and periosteal layers of the dura mater within the falx cerebri; receives most of the blood drained from the superior surface of the cerebrum and leads to the inferior jugular vein and the vertebral vein |
| Great cerebral vein | Receives most of the smaller vessels from the inferior cerebral veins and leads to the straight sinus |
| Straight sinus | Enlarged vein that drains blood from the brain; receives most of the blood from the great cerebral vein and leads to the left or right transverse sinus |
| Transverse sinuses | Pair of enlarged veins near the lambdoid suture that drains the occipital, sagittal, and straight sinuses, and leads to the sigmoid sinuses |
| Sigmoid sinuses | Enlarged vein that receives blood from the transverse sinuses and leads through the jugular foramen to the internal jugular vein |
Table 20.13
Veins Draining the Upper Limbs
The digital veins in the fingers come together in the hand to form the palmar venous arches (Figure 20.38). From here, the veins come together to form the radial vein, the ulnar vein, and the median antebrachial vein. The radial vein and the ulnar vein parallel the bones of the forearm and join together at the antebrachium to form the brachial vein, a deep vein that flows into the axillary vein in the brachium.
The basilic vein courses on the medial aspect of the antebrachium to reach the antecubital region, where it gives off a branch called the median cubital vein that crosses at an angle to join the cephalic vein. The median cubital vein is the most common site for drawing venous blood in humans. The basilic vein continues through the arm medially and superficially to the axillary vein.
The cephalic vein begins in the antebrachium and drains blood from the superficial surface of the arm into the axillary vein. It is extremely superficial and easily seen along the surface of the biceps brachii muscle in individuals with good muscle tone and in those without excessive subcutaneous adipose tissue in the arms.
As it passes through the body wall and enters the thorax, the axillary vein becomes the subclavian vein.
Many of the larger veins of the thoracic and abdominal region and upper limb are further represented in the flow chart in Figure 20.39. Table 20.14 summarizes the veins of the upper limbs.
Figure 20.38 Veins of the Upper Limb This anterior view shows the veins that drain the upper limb.
Figure 20.39 Veins Flowing into the Superior Vena Cava The flow chart summarizes the distribution of the veins flowing into the superior vena cava.
Veins of the Upper Limbs
| Vessel | Description |
| Digital veins | Drain the digits and lead to the palmar arches of the hand and dorsal venous arch of the foot |
| Palmar venous arches | Drain the hand and digits, and lead to the radial vein, ulnar veins, and the median antebrachial vein |
| Radial vein | Vein that parallels the radius and radial artery; arises from the palmar venous arches and leads to the brachial vein |
| Ulnar vein | Vein that parallels the ulna and ulnar artery; arises from the palmar venous arches and leads to the brachial vein |
| Brachial vein | Deeper vein of the arm that forms from the radial and ulnar veins in the lower arm; leads to the axillary vein |
| Basilic vein | Superficial vein of the arm that arises from the median antebrachial vein, intersects with the median cubital vein, parallels the ulnar vein, and continues into the upper arm; along with the brachial vein, it leads to the axillary vein |
| Median cubital vein | Superficial vessel located in the antecubital region that links the cephalic vein to the basilic vein in the form of a v; a frequent site from which to draw blood |
| Cephalic vein | Superficial vessel in the upper arm; leads to the axillary vein |
| Axillary vein | The major vein in the axillary region; drains the upper limb and becomes the subclavian vein |
Table 20.14
The Inferior Vena Cava
Other than the small amount of blood drained by the azygos and hemiazygos veins, most of the blood inferior to the thoracic diaphragm drains into the inferior vena cava before it is returned to the heart (see Figure 20.36). Lying just beneath the parietal peritoneum in the abdominal cavity, the inferior vena cava parallels the abdominal aorta, where it can receive blood from abdominal veins.
Blood supply from the kidneys flows into each renal vein, normally the largest veins entering the inferior vena cava. A number of other, smaller veins empty into the left renal vein. Each adrenal vein drains the adrenal or suprarenal glands located immediately superior to the kidneys. The right adrenal vein enters the inferior vena cava directly, whereas the left adrenal vein enters the left renal vein.
From the male reproductive organs, each testicular vein flows from the scrotum, forming a portion of the spermatic cord. Each ovarian vein drains an ovary in females. Each of these veins is generically called a gonadal vein. The right gonadal vein empties directly into the inferior vena cava, and the left gonadal vein empties into the left renal vein.
Blood supply from the liver drains into each hepatic vein and directly into the inferior vena cava. Since the inferior vena cava lies primarily to the right of the vertebral column and aorta, the left renal vein is longer, as are the left phrenic, adrenal, and gonadal veins. The longer length of the left renal vein makes the left kidney the primary target of surgeons removing this organ for donation. Figure 20.40 provides a flow chart of the veins flowing into the inferior vena cava. Table 20.15 summarizes the major veins of the abdominal region.
Figure 20.40 Venous Flow into Inferior Vena Cava The flow chart summarizes veins that deliver blood to the inferior vena cava.
Major Veins of the Abdominal Region
| Vessel | Description |
| Inferior vena cava | Large systemic vein that drains blood from areas largely inferior to the thoracic diaphragm; empties into the right atrium |
| Renal vein | Largest vein entering the inferior vena cava; drains the kidneys and flows into the inferior vena cava |
| Adrenal vein | Drains the adrenal or suprarenal; the right adrenal vein enters the inferior vena cava directly and the left adrenal vein enters the left renal vein |
| Testicular vein | Drains the testes and forms part of the spermatic cord; the right testicular vein empties directly into the inferior vena cava and the left testicular vein empties into the left renal vein |
| Ovarian vein | Drains the ovary; the right ovarian vein empties directly into the inferior vena cava and the left ovarian vein empties into the left renal vein |
| Gonadal vein | Generic term for a vein draining a reproductive organ; may be either an ovarian vein or a testicular vein, depending on the sex of the individual |
| Hepatic vein | Drains systemic blood from the liver and flows into the inferior vena cava |
Table 20.15
Veins Draining the Lower Limbs
The superior surface of the foot drains into the digital veins, and the inferior surface drains into the plantar veins, which flow into a complex series of anastomoses in the feet and ankles, including the dorsal venous arch and the plantar venous arch (Figure 20.41). From the dorsal venous arch, blood supply drains into the anterior and posterior tibial veins. The anterior tibial vein drains the area near the tibialis anterior muscle and combines with the posterior tibial vein and the fibular vein to form the popliteal vein. The posterior tibial vein drains the posterior surface of the tibia and joins the popliteal vein. The fibular vein drains the muscles and integument in proximity to the fibula and also joins the popliteal vein. The small saphenous vein located on the lateral surface of the leg drains blood from the superficial regions of the lower leg and foot, and flows into to the popliteal vein. As the popliteal vein passes behind the knee in the popliteal region, it becomes the femoral vein. It is palpable in patients without excessive adipose tissue.
Close to the body wall, the great saphenous vein, the deep femoral vein, and the femoral circumflex vein drain into the femoral vein. The great saphenous vein is a prominent surface vessel located on the medial surface of the leg and thigh that collects blood from the superficial portions of these areas. The deep femoral vein, as the name suggests, drains blood from the deeper portions of the thigh.
As the femoral vein penetrates the body wall from the femoral portion of the upper limb, it becomes the external iliac vein, a large vein that drains blood from the leg to the common iliac vein. The pelvic organs and integument drain into the internal iliac vein, which forms from several smaller veins in the region, including the umbilical veins that run on either side of the bladder. The external and internal iliac veins combine near the inferior portion of the sacroiliac joint to form the common iliac vein. In addition to blood supply from the external and internal iliac veins, the middle sacral vein drains the sacral region into the common iliac vein. Similar to the common iliac arteries, the common iliac veins come together at the level of L5 to form the inferior vena cava.
Figure 20.42 is a flow chart of veins flowing into the lower limb. Table 20.16 summarizes the major veins of the lower limbs.
Figure 20.41 Major Veins Serving the Lower Limbs Anterior and posterior views show the major veins that drain the lower limb into the inferior vena cava.
Figure 20.42 Major Veins of the Lower Limb The flow chart summarizes venous flow from the lower limb.
Veins of the Lower Limbs
| Vessel | Description |
| Plantar veins | Drain the foot and flow into the plantar venous arch |
| Dorsal venous arch | Drains blood from digital veins and vessels on the superior surface of the foot |
| Plantar venous arch | Formed from the plantar veins; flows into the anterior and posterior tibial veins through anastomoses |
| Anterior tibial vein | Formed from the dorsal venous arch; drains the area near the tibialis anterior muscle and flows into the popliteal vein |
| Posterior tibial vein | Formed from the dorsal venous arch; drains the area near the posterior surface of the tibia and flows into the popliteal vein |
| Small saphenous vein | Located on the lateral surface of the leg; drains blood from the superficial regions of the lower leg and foot, and flows into the popliteal vein |
| Popliteal vein | Drains the region behind the knee and forms from the fusion of the fibular, anterior, and posterior tibial veins; flows into the femoral vein |
| Great saphenous vein | Prominent surface vessel located on the medial surface of the leg and thigh; drains the superficial portions of these areas and flows into the femoral vein |
| Deep femoral vein | Drains blood from the deeper portions of the thigh and flows into the femoral vein |
| Femoral vein | Drains the upper leg; receives blood from the great saphenous vein, the deep femoral vein, and the femoral circumflex vein; becomes the external iliac vein when it crosses the body wall |
| External iliac vein | Formed when the femoral vein passes into the body cavity; drains the legs and flows into the common iliac vein |
| Internal iliac vein | Drains the pelvic organs and integument; formed from several smaller veins in the region; flows into the common iliac vein |
| Common iliac vein | Flows into the inferior vena cava at the level of L5; the left common iliac vein drains the sacral region; formed from the union of the external and internal iliac veins near the inferior portion of the sacroiliac joint |
Table 20.16
Hepatic Portal System
The liver is a complex biochemical processing plant. It packages nutrients absorbed by the digestive system; produces plasma proteins, clotting factors, and bile; and disposes of worn-out cell components and waste products. Instead of entering the circulation directly, absorbed nutrients and certain wastes (for example, materials produced by the spleen) travel to the liver for processing. They do so via the hepatic portal system (Figure 20.43). Portal systems begin and end in capillaries. In this case, the initial capillaries from the stomach, small intestine, large intestine, and spleen lead to the hepatic portal vein and end in specialized capillaries within the liver, the hepatic sinusoids. You saw the only other portal system with the hypothalamic-hypophyseal portal vessel in the endocrine chapter.
The hepatic portal system consists of the hepatic portal vein and the veins that drain into it. The hepatic portal vein itself is relatively short, beginning at the level of L2 with the confluence of the superior mesenteric and splenic veins. It also receives branches from the inferior mesenteric vein, plus the splenic veins and all their tributaries. The superior mesenteric vein receives blood from the small intestine, two-thirds of the large intestine, and the stomach. The inferior mesenteric vein drains the distal third of the large intestine, including the descending colon, the sigmoid colon, and the rectum. The splenic vein is formed from branches from the spleen, pancreas, and portions of the stomach, and the inferior mesenteric vein. After its formation, the hepatic portal vein also receives branches from the gastric veins of the stomach and cystic veins from the gall bladder. The hepatic portal vein delivers materials from these digestive and circulatory organs directly to the liver for processing.
Because of the hepatic portal system, the liver receives its blood supply from two different sources: from normal systemic circulation via the hepatic artery and from the hepatic portal vein. The liver processes the blood from the portal system to remove certain wastes and excess nutrients, which are stored for later use. This processed blood, as well as the systemic blood that came from the hepatic artery, exits the liver via the right, left, and middle hepatic veins, and flows into the inferior vena cava. Overall systemic blood composition remains relatively stable, since the liver is able to metabolize the absorbed digestive components.
Figure 20.43 Hepatic Portal System The liver receives blood from the normal systemic circulation via the hepatic artery. It also receives and processes blood from other organs, delivered via the veins of the hepatic portal system. All blood exits the liver via the hepatic vein, which delivers the blood to the inferior vena cava. (Different colors are used to help distinguish among the different vessels in the system.)
Key Terms
abdominal aorta portion of the aorta inferior to the aortic hiatus and superior to the common iliac arteries
adrenal artery branch of the abdominal aorta; supplies blood to the adrenal (suprarenal) glands
adrenal vein drains the adrenal or suprarenal glands that are immediately superior to the kidneys; the right adrenal vein enters the inferior vena cava directly and the left adrenal vein enters the left renal vein
anaphylactic shock type of shock that follows a severe allergic reaction and results from massive vasodilation
angioblasts stem cells that give rise to blood vessels
angiogenesis development of new blood vessels from existing vessels
anterior cerebral artery arises from the internal carotid artery; supplies the frontal lobe of the cerebrum
anterior communicating artery anastomosis of the right and left internal carotid arteries; supplies blood to the brain
anterior tibial artery branches from the popliteal artery; supplies blood to the anterior tibial region; becomes the dorsalis pedis artery
anterior tibial vein forms from the dorsal venous arch; drains the area near the tibialis anterior muscle and leads to the popliteal vein
aorta largest artery in the body, originating from the left ventricle and descending to the abdominal region where it bifurcates into the common iliac arteries at the level of the fourth lumbar vertebra; arteries originating from the aorta distribute blood to virtually all tissues of the body
aortic arch arc that connects the ascending aorta to the descending aorta; ends at the intervertebral disk between the fourth and fifth thoracic vertebrae
aortic hiatus opening in the diaphragm that allows passage of the thoracic aorta into the abdominal region where it becomes the abdominal aorta
aortic sinuses small pockets in the ascending aorta near the aortic valve that are the locations of the baroreceptors (stretch receptors) and chemoreceptors that trigger a reflex that aids in the regulation of vascular homeostasis
arterial circle (also, circle of Willis) anastomosis located at the base of the brain that ensures continual blood supply; formed from branches of the internal carotid and vertebral arteries; supplies blood to the brain
arteriole (also, resistance vessel) very small artery that leads to a capillary
arteriovenous anastomosis short vessel connecting an arteriole directly to a venule and bypassing the capillary beds
artery blood vessel that conducts blood away from the heart; may be a conducting or distributing vessel
ascending aorta initial portion of the aorta, rising from the left ventricle for a distance of approximately 5 cm
atrial reflex mechanism for maintaining vascular homeostasis involving atrial baroreceptors: if blood is returning to the right atrium more rapidly than it is being ejected from the left ventricle, the atrial receptors will stimulate the cardiovascular centers to increase sympathetic firing and increase cardiac output until the situation is reversed; the opposite is also true
axillary artery continuation of the subclavian artery as it penetrates the body wall and enters the axillary region; supplies blood to the region near the head of the humerus (humeral circumflex arteries); the majority of the vessel continues into the brachium and becomes the brachial artery
axillary vein major vein in the axillary region; drains the upper limb and becomes the subclavian vein
azygos vein originates in the lumbar region and passes through the thoracic diaphragm into the thoracic cavity on the right side of the vertebral column; drains blood from the intercostal veins, esophageal veins, bronchial veins, and other veins draining the mediastinal region; leads to the superior vena cava
basilar artery formed from the fusion of the two vertebral arteries; sends branches to the cerebellum, brain stem, and the posterior cerebral arteries; the main blood supply to the brain stem
basilic vein superficial vein of the arm that arises from the palmar venous arches, intersects with the median cubital vein, parallels the ulnar vein, and continues into the upper arm; along with the brachial vein, it leads to the axillary vein
blood colloidal osmotic pressure (BCOP) pressure exerted by colloids suspended in blood within a vessel; a primary determinant is the presence of plasma proteins
blood flow movement of blood through a vessel, tissue, or organ that is usually expressed in terms of volume per unit of time
blood hydrostatic pressure force blood exerts against the walls of a blood vessel or heart chamber
blood islands masses of developing blood vessels and formed elements from mesodermal cells scattered throughout the embryonic disc
blood pressure force exerted by the blood against the wall of a vessel or heart chamber; can be described with the more generic term hydrostatic pressure
brachial artery continuation of the axillary artery in the brachium; supplies blood to much of the brachial region; gives off several smaller branches that provide blood to the posterior surface of the arm in the region of the elbow; bifurcates into the radial and ulnar arteries at the coronoid fossa
brachial vein deeper vein of the arm that forms from the radial and ulnar veins in the lower arm; leads to the axillary vein
brachiocephalic artery single vessel located on the right side of the body; the first vessel branching from the aortic arch; gives rise to the right subclavian artery and the right common carotid artery; supplies blood to the head, neck, upper limb, and wall of the thoracic region
brachiocephalic vein one of a pair of veins that form from a fusion of the external and internal jugular veins and the subclavian vein; subclavian, external and internal jugulars, vertebral, and internal thoracic veins lead to it; drains the upper thoracic region and flows into the superior vena cava
bronchial artery systemic branch from the aorta that provides oxygenated blood to the lungs in addition to the pulmonary circuit
bronchial vein drains the systemic circulation from the lungs and leads to the azygos vein
capacitance ability of a vein to distend and store blood
capacitance vessels veins
capillary smallest of blood vessels where physical exchange occurs between the blood and tissue cells surrounded by interstitial fluid
capillary bed network of 10–100 capillaries connecting arterioles to venules
capillary hydrostatic pressure (CHP) force blood exerts against a capillary
cardiogenic shock type of shock that results from the inability of the heart to maintain cardiac output
carotid sinuses small pockets near the base of the internal carotid arteries that are the locations of the baroreceptors and chemoreceptors that trigger a reflex that aids in the regulation of vascular homeostasis
cavernous sinus enlarged vein that receives blood from most of the other cerebral veins and the eye socket, and leads to the petrosal sinus
celiac trunk (also, celiac artery) major branch of the abdominal aorta; gives rise to the left gastric artery, the splenic artery, and the common hepatic artery that forms the hepatic artery to the liver, the right gastric artery to the stomach, and the cystic artery to the gall bladder
cephalic vein superficial vessel in the upper arm; leads to the axillary vein
cerebrovascular accident (CVA) blockage of blood flow to the brain; also called a stroke
circle of Willis (also, arterial circle) anastomosis located at the base of the brain that ensures continual blood supply; formed from branches of the internal carotid and vertebral arteries; supplies blood to the brain
circulatory shock also simply called shock; a life-threatening medical condition in which the circulatory system is unable to supply enough blood flow to provide adequate oxygen and other nutrients to the tissues to maintain cellular metabolism
common carotid artery right common carotid artery arises from the brachiocephalic artery, and the left common carotid arises from the aortic arch; gives rise to the external and internal carotid arteries; supplies the respective sides of the head and neck
common hepatic artery branch of the celiac trunk that forms the hepatic artery, the right gastric artery, and the cystic artery
common iliac artery branch of the aorta that leads to the internal and external iliac arteries
common iliac vein one of a pair of veins that flows into the inferior vena cava at the level of L5; the left common iliac vein drains the sacral region; divides into external and internal iliac veins near the inferior portion of the sacroiliac joint
compliance degree to which a blood vessel can stretch as opposed to being rigid
continuous capillary most common type of capillary, found in virtually all tissues except epithelia and cartilage; contains very small gaps in the endothelial lining that permit exchange
cystic artery branch of the common hepatic artery; supplies blood to the gall bladder
deep femoral artery branch of the femoral artery; gives rise to the lateral circumflex arteries
deep femoral vein drains blood from the deeper portions of the thigh and leads to the femoral vein
descending aorta portion of the aorta that continues downward past the end of the aortic arch; subdivided into the thoracic aorta and the abdominal aorta
diastolic pressure lower number recorded when measuring arterial blood pressure; represents the minimal value corresponding to the pressure that remains during ventricular relaxation
digital arteries formed from the superficial and deep palmar arches; supply blood to the digits
digital veins drain the digits and feed into the palmar arches of the hand and dorsal venous arch of the foot
dorsal arch (also, arcuate arch) formed from the anastomosis of the dorsalis pedis artery and medial and plantar arteries; branches supply the distal portions of the foot and digits
dorsal venous arch drains blood from digital veins and vessels on the superior surface of the foot
dorsalis pedis artery forms from the anterior tibial artery; branches repeatedly to supply blood to the tarsal and dorsal regions of the foot
elastic artery (also, conducting artery) artery with abundant elastic fibers located closer to the heart, which maintains the pressure gradient and conducts blood to smaller branches
esophageal artery branch of the thoracic aorta; supplies blood to the esophagus
esophageal vein drains the inferior portions of the esophagus and leads to the azygos vein
external carotid artery arises from the common carotid artery; supplies blood to numerous structures within the face, lower jaw, neck, esophagus, and larynx
external elastic membrane membrane composed of elastic fibers that separates the tunica media from the tunica externa; seen in larger arteries
external iliac artery branch of the common iliac artery that leaves the body cavity and becomes a femoral artery; supplies blood to the lower limbs
external iliac vein formed when the femoral vein passes into the body cavity; drains the legs and leads to the common iliac vein
external jugular vein one of a pair of major veins located in the superficial neck region that drains blood from the more superficial portions of the head, scalp, and cranial regions, and leads to the subclavian vein
femoral artery continuation of the external iliac artery after it passes through the body cavity; divides into several smaller branches, the lateral deep femoral artery, and the genicular artery; becomes the popliteal artery as it passes posterior to the knee
femoral circumflex vein forms a loop around the femur just inferior to the trochanters; drains blood from the areas around the head and neck of the femur; leads to the femoral vein
femoral vein drains the upper leg; receives blood from the great saphenous vein, the deep femoral vein, and the femoral circumflex vein; becomes the external iliac vein when it crosses the body wall
fenestrated capillary type of capillary with pores or fenestrations in the endothelium that allow for rapid passage of certain small materials
fibular vein drains the muscles and integument near the fibula and leads to the popliteal vein
filtration in the cardiovascular system, the movement of material from a capillary into the interstitial fluid, moving from an area of higher pressure to lower pressure
foramen ovale shunt that directly connects the right and left atria and helps to divert oxygenated blood from the fetal pulmonary circuit
gonadal artery branch of the abdominal aorta; supplies blood to the gonads or reproductive organs; also described as ovarian arteries or testicular arteries, depending upon the sex of the individual
gonadal vein generic term for a vein draining a reproductive organ; may be either an ovarian vein or a testicular vein, depending on the sex of the individual
great cerebral vein receives most of the smaller vessels from the inferior cerebral veins and leads to the straight sinus
great saphenous vein prominent surface vessel located on the medial surface of the leg and thigh; drains the superficial portions of these areas and leads to the femoral vein
hemangioblasts embryonic stem cells that appear in the mesoderm and give rise to both angioblasts and pluripotent stem cells
hemiazygos vein smaller vein complementary to the azygos vein; drains the esophageal veins from the esophagus and the left intercostal veins, and leads to the brachiocephalic vein via the superior intercostal vein
hepatic artery proper branch of the common hepatic artery; supplies systemic blood to the liver
hepatic portal system specialized circulatory pathway that carries blood from digestive organs to the liver for processing before being sent to the systemic circulation
hepatic vein drains systemic blood from the liver and flows into the inferior vena cava hypertension chronic and persistent blood pressure measurements of 140/90 mm Hg or above hypervolemia abnormally high levels of fluid and blood within the body
hypovolemia abnormally low levels of fluid and blood within the body
hypovolemic shock type of circulatory shock caused by excessive loss of blood volume due to hemorrhage or possibly dehydration
hypoxia lack of oxygen supply to the tissues
inferior mesenteric artery branch of the abdominal aorta; supplies blood to the distal segment of the large intestine and rectum
inferior vena cava large systemic vein that drains blood from areas largely inferior to the diaphragm; empties into the right atrium
intercostal artery branch of the thoracic aorta; supplies blood to the muscles of the thoracic cavity and vertebral column
internal carotid artery arises from the common carotid artery and begins with the carotid sinus; goes through the carotid canal of the temporal bone to the base of the brain; combines with branches of the vertebral artery forming the arterial circle; supplies blood to the brain
internal elastic membrane membrane composed of elastic fibers that separates the tunica intima from the tunica media; seen in larger arteries
internal iliac artery branch from the common iliac arteries; supplies blood to the urinary bladder, walls of the pelvis, external genitalia, and the medial portion of the femoral region; in females, also provide blood to the uterus and vagina
internal iliac vein drains the pelvic organs and integument; formed from several smaller veins in the region; leads to the common iliac vein
internal jugular vein one of a pair of major veins located in the neck region that passes through the jugular foramen and canal, flows parallel to the common carotid artery that is more or less its counterpart; primarily drains blood from the brain, receives the superficial facial vein, and empties into the subclavian vein
internal thoracic artery (also, mammary artery) arises from the subclavian artery; supplies blood to the thymus, pericardium of the heart, and the anterior chest wall
internal thoracic vein (also, internal mammary vein) drains the anterior surface of the chest wall and leads to the brachiocephalic vein
interstitial fluid colloidal osmotic pressure (IFCOP) pressure exerted by the colloids within the interstitial fluid
interstitial fluid hydrostatic pressure (IFHP) ischemia insufficient blood flow to the tissues force exerted by the fluid in the tissue spaces
Korotkoff sounds noises created by turbulent blood flow through the vessels
lateral plantar artery arises from the bifurcation of the posterior tibial arteries; supplies blood to the lateral plantar surfaces of the foot
left gastric artery branch of the celiac trunk; supplies blood to the stomach
lumbar arteries branches of the abdominal aorta; supply blood to the lumbar region, the abdominal wall, and spinal cord
lumbar veins drain the lumbar portion of the abdominal wall and spinal cord; the superior lumbar veins drain into the azygos vein on the right or the hemiazygos vein on the left; blood from these vessels is returned to the superior vena cava rather than the inferior vena cava
lumen interior of a tubular structure such as a blood vessel or a portion of the alimentary canal through which blood, chyme, or other substances travel
maxillary vein drains blood from the maxillary region and leads to the external jugular vein
mean arterial pressure (MAP) average driving force of blood to the tissues; approximated by taking diastolic pressure and adding 1/3 of pulse pressure
medial plantar artery arises from the bifurcation of the posterior tibial arteries; supplies blood to the medial plantar surfaces of the foot
median cubital vein superficial vessel located in the antecubital region that links the cephalic vein to the basilic vein in the form of a v; a frequent site for a blood draw
median sacral artery continuation of the aorta into the sacrum
mediastinal artery branch of the thoracic aorta; supplies blood to the mediastinum metarteriole short vessel arising from a terminal arteriole that branches to supply a capillary bed microcirculation blood flow through the capillaries
middle cerebral artery another branch of the internal carotid artery; supplies blood to the temporal and parietal lobes of the cerebrum
muscular artery (also, distributing artery) artery with abundant smooth muscle in the tunica media that branches to distribute blood to the arteriole network
myogenic response constriction or dilation in the walls of arterioles in response to pressures related to blood flow; reduces high blood flow or increases low blood flow to help maintain consistent flow to the capillary network
nervi vasorum small nerve fibers found in arteries and veins that trigger contraction of the smooth muscle in their walls
net filtration pressure (NFP) force driving fluid out of the capillary and into the tissue spaces; equal to the difference of the capillary hydrostatic pressure and the blood colloidal osmotic pressure
neurogenic shock type of shock that occurs with cranial or high spinal injuries that damage the cardiovascular centers in the medulla oblongata or the nervous fibers originating from this region
obstructive shock type of shock that occurs when a significant portion of the vascular system is blocked
occipital sinus enlarged vein that drains the occipital region near the falx cerebelli and flows into the left and right transverse sinuses, and also into the vertebral veins
ophthalmic artery branch of the internal carotid artery; supplies blood to the eyes
ovarian artery branch of the abdominal aorta; supplies blood to the ovary, uterine (Fallopian) tube, and uterus
ovarian vein drains the ovary; the right ovarian vein leads to the inferior vena cava and the left ovarian vein leads to the left renal vein
palmar arches superficial and deep arches formed from anastomoses of the radial and ulnar arteries; supply blood to the hand and digital arteries
palmar venous arches drain the hand and digits, and feed into the radial and ulnar veins
parietal branches (also, somatic branches) group of arterial branches of the thoracic aorta; includes those that supply blood to the thoracic cavity, vertebral column, and the superior surface of the thoracic diaphragm
perfusion distribution of blood into the capillaries so the tissues can be supplied
pericardial artery branch of the thoracic aorta; supplies blood to the pericardium
petrosal sinus enlarged vein that receives blood from the cavernous sinus and flows into the internal jugular vein
phrenic vein drains the thoracic diaphragm; the right phrenic vein flows into the inferior vena cava and the left phrenic vein leads to the left renal vein
plantar veins drain the foot and lead to the plantar venous arch
plantar venous arch formed from the plantar veins; leads to the anterior and posterior tibial veins through anastomoses
popliteal artery continuation of the femoral artery posterior to the knee; branches into the anterior and posterior tibial arteries
popliteal vein continuation of the femoral vein behind the knee; drains the region behind the knee and forms from the fusion of the fibular and anterior and posterior tibial veins
posterior cerebral artery branch of the basilar artery that forms a portion of the posterior segment of the arterial circle; supplies blood to the posterior portion of the cerebrum and brain stem
posterior communicating artery branch of the posterior cerebral artery that forms part of the posterior portion of the arterial circle; supplies blood to the brain
posterior tibial artery branch from the popliteal artery that gives rise to the fibular or peroneal artery; supplies blood to the posterior tibial region
posterior tibial vein forms from the dorsal venous arch; drains the area near the posterior surface of the tibia and leads to the popliteal vein
precapillary sphincters circular rings of smooth muscle that surround the entrance to a capillary and regulate blood flow into that capillary
pulmonary artery one of two branches, left and right, that divides off from the pulmonary trunk and leads to smaller arterioles and eventually to the pulmonary capillaries
pulmonary circuit system of blood vessels that provide gas exchange via a network of arteries, veins, and capillaries that run from the heart, through the body, and back to the lungs
pulmonary trunk single large vessel exiting the right ventricle that divides to form the right and left pulmonary arteries
pulmonary veins two sets of paired vessels, one pair on each side, that are formed from the small venules leading away from the pulmonary capillaries that flow into the left atrium
pulse alternating expansion and recoil of an artery as blood moves through the vessel; an indicator of heart rate
pulse pressure difference between the systolic and diastolic pressures
radial artery formed at the bifurcation of the brachial artery; parallels the radius; gives off smaller branches until it reaches the carpal region where it fuses with the ulnar artery to form the superficial and deep palmar arches; supplies blood to the lower arm and carpal region
radial vein parallels the radius and radial artery; arises from the palmar venous arches and leads to the brachial vein
reabsorption in the cardiovascular system, the movement of material from the interstitial fluid into the capillaries
renal artery branch of the abdominal aorta; supplies each kidney
renal vein largest vein entering the inferior vena cava; drains the kidneys and leads to the inferior vena cava
resistance any condition or parameter that slows or counteracts the flow of blood
respiratory pump increase in the volume of the thorax during inhalation that decreases air pressure, enabling venous blood to flow into the thoracic region, then exhalation increases pressure, moving blood into the atria
right gastric artery branch of the common hepatic artery; supplies blood to the stomach
sepsis (also, septicemia) organismal-level inflammatory response to a massive infection
septic shock (also, blood poisoning) type of shock that follows a massive infection resulting in organism-wide inflammation
sigmoid sinuses enlarged veins that receive blood from the transverse sinuses; flow through the jugular foramen and into the internal jugular vein
sinusoid capillary rarest type of capillary, which has extremely large intercellular gaps in the basement membrane in addition to clefts and fenestrations; found in areas such as the bone marrow and liver where passage of large molecules occurs
skeletal muscle pump effect on increasing blood pressure within veins by compression of the vessel caused by the contraction of nearby skeletal muscle
small saphenous vein located on the lateral surface of the leg; drains blood from the superficial regions of the lower leg and foot, and leads to the popliteal vein
sphygmomanometer blood pressure cuff attached to a device that measures blood pressure
splenic artery branch of the celiac trunk; supplies blood to the spleen
straight sinus enlarged vein that drains blood from the brain; receives most of the blood from the great cerebral vein and flows into the left or right transverse sinus
subclavian artery right subclavian arises from the brachiocephalic artery, whereas the left subclavian artery arises from the aortic arch; gives rise to the internal thoracic, vertebral, and thyrocervical arteries; supplies blood to the arms, chest, shoulders, back, and central nervous system
subclavian vein located deep in the thoracic cavity; becomes the axillary vein as it enters the axillary region; drains the axillary and smaller local veins near the scapular region; leads to the brachiocephalic vein
subscapular vein drains blood from the subscapular region and leads to the axillary vei
superior mesenteric artery branch of the abdominal aorta; supplies blood to the small intestine (duodenum, jejunum, and ileum), the pancreas, and a majority of the large intestine
superior phrenic artery branch of the thoracic aorta; supplies blood to the superior surface of the thoracic diaphragm
superior sagittal sinus enlarged vein located midsagittally between the meningeal and periosteal layers of the dura mater within the falx cerebri; receives most of the blood drained from the superior surface of the cerebrum and leads to the inferior jugular vein and the vertebral vein
superior vena cava large systemic vein; drains blood from most areas superior to the thoracic diaphragm; empties into the right atrium
systolic pressure larger number recorded when measuring arterial blood pressure; represents the maximum value following ventricular contraction
temporal vein drains blood from the temporal region and leads to the external jugular vein
testicular artery branch of the abdominal aorta; will ultimately travel outside the body cavity to the testes and form one component of the spermatic cord
testicular vein drains the testes and forms part of the spermatic cord; the right testicular vein empties directly into the inferior vena cava and the left testicular vein empties into the left renal vein
thoracic aorta portion of the descending aorta superior to the aortic hiatus
thoroughfare channel continuation of the metarteriole that enables blood to bypass a capillary bed and flow directly into a venule, creating a vascular shunt
thyrocervical artery arises from the subclavian artery; supplies blood to the thyroid, the cervical region, the upper back, and shoulder
transient ischemic attack (TIA) temporary loss of neurological function caused by a brief interruption in blood flow; also known as a mini-stroke
transverse sinuses pair of enlarged veins near the lambdoid suture that drain the occipital, sagittal, and straight sinuses, and leads to the sigmoid sinuses
trunk large vessel that gives rise to smaller vessels
tunica externa (also, tunica adventitia) outermost layer or tunic of a vessel (except capillaries)
tunica intima (also, tunica interna) innermost lining or tunic of a vessel
tunica media middle layer or tunic of a vessel (except capillaries)
ulnar artery formed at the bifurcation of the brachial artery; parallels the ulna; gives off smaller branches until it reaches the carpal region where it fuses with the radial artery to form the superficial and deep palmar arches; supplies blood to the lower arm and carpal region
ulnar vein parallels the ulna and ulnar artery; arises from the palmar venous arches and leads to the brachial vein
umbilical vein single vessel that originates in the placenta and runs within the umbilical cord, carrying oxygen- and nutrient-rich blood to the fetal heart
vasa vasorum small blood vessels located within the walls or tunics of larger vessels that supply nourishment to and remove wastes from the cells of the vessels
vascular shock type of shock that occurs when arterioles lose their normal muscular tone and dilate dramatically
vascular shunt continuation of the metarteriole and thoroughfare channel that allows blood to bypass the capillary beds to flow directly from the arterial to the venous circulation
vascular tone contractile state of smooth muscle in a blood vessel
vascular tubes rudimentary blood vessels in a developing fetus
vasoconstriction constriction of the smooth muscle of a blood vessel, resulting in a decreased vascular diameter
vasodilation relaxation of the smooth muscle in the wall of a blood vessel, resulting in an increased vascular diameter
vasomotion irregular, pulsating flow of blood through capillaries and related structures
vein blood vessel that conducts blood toward the heart
venous reserve volume of blood contained within systemic veins in the integument, bone marrow, and liver that can be returned to the heart for circulation, if needed
venule small vessel leading from the capillaries to veins
vertebral artery arises from the subclavian artery and passes through the vertebral foramen through the foramen magnum to the brain; joins with the internal carotid artery to form the arterial circle; supplies blood to the brain and spinal cord
vertebral vein arises from the base of the brain and the cervical region of the spinal cord; passes through the intervertebral foramina in the cervical vertebrae; drains smaller veins from the cranium, spinal cord, and vertebrae, and leads to the brachiocephalic vein; counterpart of the vertebral artery
visceral branches branches of the descending aorta that supply blood to the viscera
Interactive Link Questions
1. Watch this video (http://openstaxcollege.org/l/ capillaryfunct) to explore capillaries and how they function in the body. Capillaries are never more than 100 micrometers away. What is the main component of interstitial fluid?
Review Questions
Critical Thinking Questions
- Arterioles are often referred to as resistance vessels. Why?
- Cocaine use causes vasoconstriction. Is this likely to increase or decrease blood pressure, and why?
- A blood vessel with a few smooth muscle fibers and connective tissue, and only a very thin tunica externa conducts blood toward the heart. What type of vessel is this?
- You measure a patient’s blood pressure at 130/85 mm Hg. Calculate the patient’s pulse pressure and mean arterial pressure. Determine whether each pressure is low, normal, or high.
- An obese patient comes to the clinic complaining of swollen feet and ankles, fatigue, shortness of breath, and often feeling “spaced out.” She is a cashier in a grocery store, a job that requires her to stand all day. Outside of work, she engages in no physical activity. She confesses that, because of her weight, she finds even walking uncomfortable. Explain how the skeletal muscle pump might play a role in this patient’s signs and symptoms.
- A patient arrives at the emergency department with dangerously low blood pressure. The patient’s blood colloid osmotic pressure is normal. How would you expect this situation to affect the patient’s net filtration pressure?
- True or false? The plasma proteins suspended in blood cross the capillary cell membrane and enter the tissue fluid via facilitated diffusion. Explain your thinking.
- A patient arrives in the emergency department with a blood pressure of 70/45 mm Hg confused and complaining of thirst. Why?
- Identify the ventricle of the heart that pumps oxygen-depleted blood and the arteries of the body that carry oxygen-depleted blood.
- What organs do the gonadal veins drain?
- What arteries play the leading roles in supplying blood to the brain? (Figure_20.2)
The internal lining of blood and lymphatic vessels, comprised of a specific type of epithelial cells called endothelial cells.
An abnormal increase in hemoglobin concentration in the blood, caused by reduced plasma volume or increased erythrocyte numbers.
Reduced hemoglobin level in the blood due to a reduction in the number or quality of erythrocytes in the blood.
A cellular process in which substances are brought into the cell via invagination of the plasma membrane and vesicle formation.
A cellular process in which substances are transported out of the cell via fusion of packaging vesicles with the plasma membrane.